What Is Gas Chromatography?
Gas Chromatography or Gas Liquid Chromatography is a technique applied for separation, identification and quantification of components of a mixture of organic compounds by selective partitioning between the stationary phase and mobile phase inside a column followed by sequential elution of separated components. The technique is suitable for separation of compounds having following characteristics :
- High volatility
- Thermal stability
- Low molecular weights

Purpose of gas chromatography
The main purpose of the gas chromatography technique is to separate the compounds that possess:
- High volatility
- Low molecular weights
- Thermal stability
How does gas chromatography work?
For having a hold on how does chromatography works, we need to be aware of the individual components of a GC chromatogram or GC Chromatograph.
The main components are:
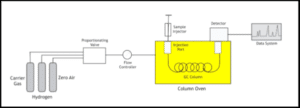
Mobile phase
In gas chromatography, usually, three types of gases are employed namely –
- Carrier gas – This is needed for the transfer of the injected sample to the separation column. They are also responsible for the subsequent transfer of separated components to the detector.Common examples: Nitrogen, helium, or hydrogen
- Fuel gas – They support the flame in Flame ionization detector (FID) detector such as Hydrogen.
- Zero air – These are the purified air that plays the role of oxidant to support the combustion of flame in the detector. Before being led to the gas chromatographic system, the above three are intermixed in the desired proportion.
Sample injector
The injector is a heated block where the sample is injected. Through the carrier gas stream, the sample is spontaneously vaporized and led to the column.
With the help of a gas-tight syringe, the liquid sample mixtures are injected whereas, with the help of automated injection valves, the gaseous mixtures are injected.
Column
This is filled with the stationary phase or its walls are covered with a liquid adsorbent. This is done for selective absorbance and retention of the sample components.
Commonly used: Packed columns and Capillary columns (More popular)
Component of a Column – Oven
The column is enclosed by a column oven which is responsible for maintaining a constant temperature during isothermal operation. This temperature when temperature programming is needed can be increased in a controlled way for acquiring effective separation of mixture components possessing different volatilities.
Detector
This is employed for the identification and quantification of components.
Here, the regions of individual peaks created relate to their concentrations and their retention times are representative of their identity.
Common examples: Flame ionization detector, Thermal conductivity detector (TCD), and Electron capture detector (ECD).
Data system
It is a set of dedicated software that provides control over many important operational parameters like injection sequence, wash cycles, over-temperature control, the flow rate of gases, detector wavelength, etc. Simultaneously, the data station calculates and displays the parameters.
Figure: Systematic Diagram of GC
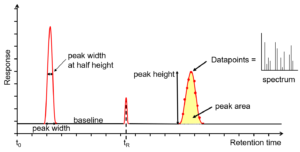
Gas chromatographic analysis
The X-axis – Retention time of peak (Rt)
This is calculated from the time the sample was injected into the column (t0) till it reaches the detector. Every analyte peak has a retention time that is measured from the apex of the peak, just like tR.
The Y-axis – Detector response
This shows the measured response of the analyte peak within the detector.
The baseline here represents the signal received from the detector where no analyte is eluting from the column or is beneath the detection limit. It is considered as an indication of a problem or indication to check the maintenance, in situations where the baseline is found higher than usual.
Measurements such as width at the baseline, width at half height, area, and total height can be withdrawn from the peak.
For better sensitivity and better resolution, narrower, sharper peaks are desired.
The accuracy of measurements is influenced by the total number of data points present across a peak.
FIGURE: Chromatogram output from GC. Image courtesy: Anthias Consulting.
Types
Majorly, there are two gas chromatography types into which it is classified – GLC or gas-liquid chromatography and GSC or gas-solid chromatography.
Both the methods use either liquid or solid as a stationary phase while using gas as the mobile phase. In Gas-solid chromatography, the retention of analytes is due to physical adsorption. On the other hand, gas-liquid chromatography separates the ions or molecules that are dissolved in a solvent.
The underlying principle is – as the sample solution makes contact with the second solid or liquid phase, the solutes will start interacting with the other phases. Due to different adsorption rates, ion-exchanges, partitioning or sizes, the interaction will vary, and that’s what will enable the separation of the mixed components from each other. These differences will make the sample mixture pass at different rates through the column, and the compounds can be separated.
A Gas Chromatograph like any other analytical instrument has evolved from one with several knobs and dials to one having a simple microprocessor-based keypad to control the operational parameters.
The simplification has resulted in ease of operation and time-saving. An understanding of the main component parts will help in maximum utilization of system capabilities.
Dimensions of Gas Chromatography
Gas Chromatography has a high peak capacity in comparison to other separation techniques. Although it has the ability to separate a huge number of compounds, there are a few applications that require thousands of peaks to be separated and we don’t have enough theoretical plates to separate them through chromatography.
A common example of this is the analysis of diesel that involves identifying trace analytes in complex matrices, such as food samples or environmental samples.
The analysis can be carried out without full chromatographic resolution through spectral resolution, where MS is hyphenated with GC. However, this technique can be successful under the condition that the coeluting peaks have different spectra.
Heart-cutting works well where the majority of the peaks are separated through a column and then a few groups of coeluting peaks are cut and transferred to a new column consisting of different stationary phase and selectivity. If the samples are complex with frequent co-elutions, two-dimensional chromatography is used.
Gas chromatography applications
Since the discovery of the gas chromatographic system, the areas of Gas chromatography applications is ever-increasing which includes:
- Pharmaceutical industry
- Research
- Medical and Forensic
- Environmental monitoring (both inside laboratories, and natural water bodies)
- Petroleum refining and petrochemicals
- Edible oils
- Flavors, beverages, and the food industry
- Fragrance industry (Cosmetics)
- Polymers and plastics
- Pesticides
Gas Chromatography: Limitations and Common Issues
Limitations
Gas chromatography is broadly utilized across many industries for routine analysis, research or analysing hundreds and thousands of compounds in different samples and components from solids to gases. This technique is quite robust and can be easily mixed or coupled with other distinctive techniques, such as mass spectrometry.
However, gas chromatography can analyse volatile compounds from helium/hydrogen only when their molecular weights are around 1250 u. In the case of compounds that are thermally labile, exposure to high temperatures in GC can degrade them.
Cold injection techniques and low temperatures can be used to minimize that. To prevent polar analytes from getting lost or stuck in GC, the system must be well-maintained and the analytes must be derivatized.
Issues
One of the major problems with gas chromatography is leakage. As the mobile phase is a gas that flows through the system, leakage may occur. Therefore, it is crucial to ensure that the parts and consumables are correctly installed and the system is regularly checked for leakage.
Another problem is the activity for more polar analytes, especially the ones at trace levels. Issues like irreversible adsorption or reactant breakdown can also occur due to dirt build-up in the system and silanol groups on the glass liners and columns.
Most problems are seen on the inlet area where the sample is injected, transferred and vaporized into the GC column. Hence, ensuring proper maintenance of the inlet and use of correct consumable is essential.
Glossary of GC terms
The glossary will help you familiarize with the terminology in case you are not already familiar with the gas chromatography working technique.
| Stationary Phase | A solid phase which absorbs the sample components and later releases them in a sequential manner |
|---|---|
| Mobile Phase | A stream of carrier gas used for transporting sample from injection port to the column to the detector |
| Column Oven | A compartment inside which the column is mounted. It maintains a constant temperature or a varying temperature in response to a set temperature programme. |
| Detector | A device which gives the signal response in terms of area counts under a peak |
| Column Efficiency | Expressed in terms of HETP expresses the resolving power of the GC column |
| Packed Column | A steel or glass tube wound as a coil which holds the stationary phase |
| Capillary Column | A fused silica capillary column that holds the liquid absorbent on the tube on its walls |
| Autosampler | A device capable of holding several samples, standard vials and automatically injects a predetermined sample volume into the gas chromatograph |
| Injector | Manual or automated device for precise sample volume introduction |
| FID | Flame Ionisation detector which responds to most organic compounds |
| TCD | Thermal Conductivity detector. Universal and nondestructive detector |
| ECD | Electron Capture detector. For compounds containing electronegative elements such as halogens |
| NPD | Nitrogen Phosphorus detector. Specific for compounds containing nitrogen or phosphorus |
| FPD | Flame photometric detector.Specific for sulphur and phosphorus containing compounds |
| MSD | Mass Selective detector |
| GC – MS | Hyphenated technique using a combination of GC and Maas spectrometer |
| Fronting | Distortion of peak where the peak front appears distorted |
| Peak Tailing | Distortion of peak where the tailing end of the peak appears distorted |
| Heart Cutting | A method which employs two columns of different selectivity. A selected portion of effluent from first column is passed to the second column |
| Temperature Programming | Changing temperature of column oven in a predetermined manner using a program |
| Retention Time | Time between injection and the maximum of the peak response |
| Syringe | Hand held device capable of injecting selected volume into the chromatograph |
| HETP | Height Equivalent to a theoretical plate. It is a measure of column efficiency and is expressed as an numerical value without units H = L/N The larger the number of theoretical plates the lower is HETP and better is the column efficiency |
| Septa | Rubber or silicone discs which are used inside the injector for introduction of sample into the chromatographic system. The syringe needle penetrates this disc at the time of sample injection |
| Ferrule | A plug made from graphite or grass for holding the column gas tight into the oven |
| Gas Regulator | Device comprising of a controller to record and control pressure in the gas line and also monitor the pressure inside the cylinder |
| Gas Filter | A wall mounted assembly comprising of packed cartridges capable of removing moisture, hydrocarbons, oxygen and other impurities from the inlet gases |
| PLOT | Porous Layer Open Tubular column where an absorbent is bonded to the inner surface of the column. Useful for analysis of permanent gases or high volatility liquids. |
| SCOT | Support Coated Open Tubular column. A liquid stationary phase is supported on a solid support which is coated to the inner surface of the capillary common. |
| Split Injection | Injection mode where a portion of the vaporized sample is vented out and only a small portion enters the column head. This is used for highly concentrated samples |
| Splitless Injection | Sample injection where purge valve is closed and the entire sample enters the column. The purge valve is then opened to flush the injector |
| WCOT | Wall Coated Open Tubular column. The stationary phase is bonded to the inside wall of the capillary column |
| On-Column Injection | The syringe needle enters and delivers the sample onto the top of the column head |
| Leak test | A process to establish that all connections are leak free |
| Pre-vent | A design of sample inlet that splits the injected sample and vents out a portion. The residual portion is only directed to the column. This is suitable when samples are highly concentrated |
Refresh your basic skills by registering for the free e-course on GC which will provide you an introduction to the technique and even prepare you for an interview if you are applying for a job in a laboratory equipped with a GC system.
Sign Up Now!
Want to read all the AAS free course modules right now? Here are all links to all the modules for you!
- Module 1 : Introduction to Gas Chromatography Course and its Objectives
- Module 2 : Evolution of Gas Chromatography
- Module 3 : Introduction to Gas Chromatography and Its Parts
- Module 4 : Role of Gases in Gas Chromatography
- Module 5 : Types of Gas Chromatography Injectors
- Module 6 : Types of Gas Chromatography Columns
- Module 7 : Types of Stationary Phases
- Module 8 : Types of Gas Chromatography Detectors
- Module 9 : Gas Chromatography Applications
- Module 10 : Top 10 Interview Questions on Gas Chromatography
Library of Published Articles
See the list of published articles related to GC specially prepared for upgradation of your laboratory skills and bring about exposure to new concepts and developments. You willl find that the list is ever growing with inclusion of new published articles as and when they are published.
- Understanding the response factors of a GC detector
- Selecting a suitable detector for Gas Chromatographic analysis
- Influence of changes in operational conditions on Gas Chromatograms
- Improve reproducibility of syringe injections for GC analysis
- Simple steps to minimize Column bleed from GC columns
- Sampling of Gases for analysis by Gas Chromatography
- Benefits offered by automated injection in Gas Chromatography
- How to minimize retention time drifts in Gas Chromatography?
- Familiarize with the Gas Chromatograph
- Tips on improving accuracy and precision of Gas Chromatographic injections
- Troubleshooting Tips for Gas Chromatographic Syringe
- Certificate Course on Gas Chromatography now available!
- Recommendations for switching from Gas cylinders to Laboratory gas generators
- What is Multi- dimensional GC?
- How to prevent damage to Capillary GC columns
- Benefits of Automated replacement of GC liners
- Types of Liners and their Selection
- Sample Injection Techniques for Capillary Column Gas Chromatography
- Minimization of influence of random fluctuations in operating conditions in GC analysis
- Why very long capillary GC columns are not preferred?
- Sample Injection Practices in Gas Chromatography
- How Gas Chromatography can contribute to your career prospects?
- How detector characteristics influence Gas Chromatographic response?
- Peak Height or Peak Area? – Which is the right choice for quantitative chromatographic calculations
- Importance of Colour Coding for Gas Cylinders and Lines in Laboratories
- 10 Similarities between High Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC)
- How are Gas Chromatography (GC) and High Performance Liquid Chromatography(HPLC) different?
- Advantages of Gas Chromatography (GC) over Thin Layer Chromatography (TLC)
- How is Gas Solid Chromatography different from Gas Liquid Chromatography?
- Useful Tips for Extending the useful life of a GC Capillary Column
- Now GC Method Optimization and Development on your fingertips!
- Temperature Control of the Gas Chromatographic Column
- Factors Governing the Resolution of peaks in the Gas Chromatogram
- How to save your time by preventing Gas leaks before running the Gas Chromatograph?
- Importance of Gas leak detection before starting Gas Chromatographic Analysis
- Gas Chromatography in Petroleum Refining Industry
- Chromatographic Techniques for Analysis of Flavours in Foods
- Useful tips on Handling and Care of GC Capillary Columns
- Which type of gas regulator is suitable for Gas Chromatography?
- Why Capillary Columns are preferred over Packed Columns in Gas Chromatography
- Hydrogen, Helium or Nitrogen – Which is most suitable as a Carrier Gas?
- How to handle Gas Chromatographic Gases Safely?
- How Derivatisation is useful in GC analysis?
- How to Choose a Gas Chromatographic Detector for my Analysis?
- Why is Leak checking necessary before starting Gas Chromatography analysis?
- GC Free E-Course : Introduction to Gas Chromatography
- Benefits of Split /Splitless Injection in Capillary Gas Chromatography
- Why is Conditioning necessary for GC Columns?
- Four Key Considerations for choice of Capillary Gas Chromatographic Columns
- Gas Purification Requirements in Gas Chromatography
- Factors Governing Choice of Sample Injection Syringe in Gas Chromatography
- Why are HPLC columns shorter than GC columns?
- Know your GC Chromatogram
- The results are in – Gas Chromatography is the next free e-course!
- Gas Chromatography

[…] chromatography is mainly defined as GC, and defined as an important technique for identifying and separating volatile liquids or gases […]
Want to join this course.
It was fantastic!
You are welcome.