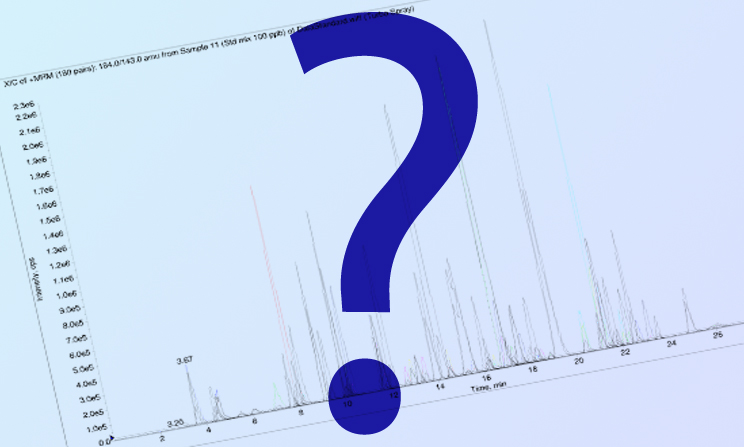
Factors Governing the Resolution of peaks in the Gas Chromatogram
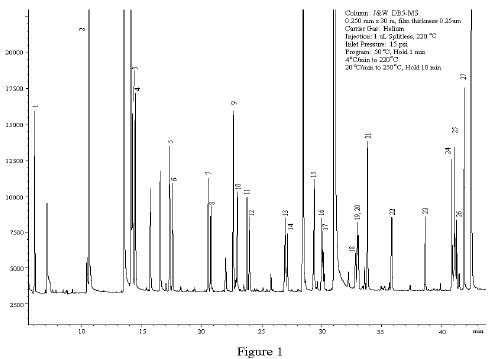
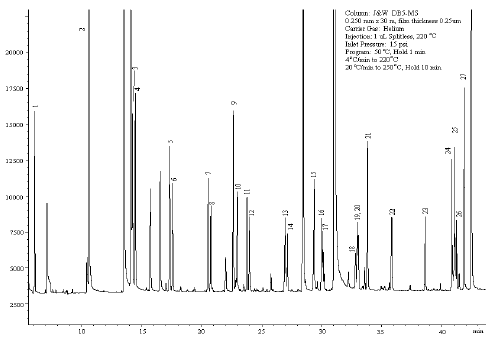
As a Gas Chromatographer your key objective is to get the best possible resolution of component peaks of the injected sample. Have you ever pondered over the various factors that can lead you to the required resolution.The operational parameters can be optimized to your advantage to get the best output from your gas chromatograph.
Boiling Point
Boiling point is the temperature at which a liquid transforms into vapour under existing pressure conditions. The lower the boiling point, the lower will be the temperature of vapour formation and shorter will be the retention time of the eluting peak. The greater the difference between the boiling points of the constituents the better will be the resolution between the separated peaks.
Column Temperature
As the column temperature increases vapour inside becomes less viscous and travels faster which in turn reduces the retention times but on the other hand leads to poor separation between the peaks. In other words the temperature of the column should be above the highest boiling point of sample components but not too high so that all the components do get sufficient time to interact with the stationary phase and elute as well resolved peaks.
Polarity
It is generally observed that compounds with similar polarities have greater affinities between themselves. Molecules having polarities similar to the stationary phase material will be retained longer due to strong interactions and vice versa.
Carrier Gas Flow Rate
At higher flow rates the carrier gas moves the sample components at a faster rate through the column and thereby reduces the retention times but though the analysis time is reduced the resolution between the peaks deteriorates as the components get limited time to interact with the stationary phase
Column Length
An increase in column length improves the resolution between the separating components. However, peak broadening arises due to increased longitudinal sample vapour diffusion inside the column.
Column Diameter
Sample concentration decides the choice of column diameter. Loss of resolution, poor reproducibility and peak distortions result if sample loading exceeds column capacity. Typical sample loading can range from 10 ng for 0.1mm id to 2000 ng for 0.53 mm id columns. Narrow columns improve resolution but the loading capacity decreases correspondingly.
Film Thickness
The film thickness of bound liquids on the stationary phase usually ranges between 0.1 to 5.0 μm. Thicker films can be used for increased sample loading and increased residence time for highly volatile components to prevent co-elution. However, thicker films result in high retention times and higher elution temperatures. Choice of optimum film thickness can result in better separation of the sample constituents.
As you can see there are a number of factors that contribute to resolution between the constituents of a sample and method development involves a careful optimization of the operational parameters to achieve the desired results.

Thanks for sharing important details like this. I enjoyed reading your article, and love to know the latest updates.