Atomic Absorption Spectroscopy is an instrumental analysis technique for rapid trace metal analysis.It is based on element specific wavelength light absorption by ground state atoms in the flame or electrothermal graphite furnace.
It finds immense applications in the analysis for trace metals in soils, lakes, rivers, oceans, and drinking water, pharmaceuticals, foods and beverages, geological and mineralogical samples, petroleum products, biological fluids and specimens and forensic analysis. It is common to get results in ppm levels and a higher sensitivity of ppb levels when we using graphite furnace atomisation.
Why not start with a short video?
An illustrated video will depict the changes that take place when a sample containing a trace metal is aspirated into a flame. Such physical changes are accompanied by changes in absorption of light by ground state atoms and measurement of absorption signal for quantitative estimations is illustrated in the video.
Uses of Atomic Absorption Spectroscopy
Atomic Absorption Spectroscopy provides cost-effective viable solutions for the analysis of trace amounts of metals in the entire range of natural and manmade materials such as Geological samples, Environmental samples, Biological Specimens, Agricultural produce and soils, Pharmaceuticals, Foods and Drinking water.
The technique affords advantages of speed, sensitivity and precision over the classical gravimetric methods. Introduction of accessories such as graphite furnace, flow injection analysis and improvements in the suppression of matrix interferences have further contributed to improvement in sensitivity and selectivity of analytes in complex matrices.
Atomic Absorption Spectroscopy applications in the field of environment, drinking water, mining and mineralogy, oceanographic studies, soils, pharmaceuticals, foods, toys, forensic investigations are of great significance.
The list is endless and presence or absence of trace metals is a factor that cannot be overlooked for evaluation of characteristics of materials or concerns regarding human health and safety.
The chemical techniques used for the analysis of trace metals have evolved from simple gravimetric methods to highly sophisticated time saving instrumental techniques. Atomic Absorption Spectroscopy is a popular technique which involves moderate investment and affordable operational cost.
These features coupled with a high degree of accuracy and precision of results has contributed to the widespread presence of atomic absorption spectrometers in college laboratories, industrial laboratories and regulatory body laboratories across the world.
Principle of Atomic Absorption Spectroscopy
Atomic absorption spectroscopy (AAS) is based upon the principle that free atoms in the ground state can absorb light of a certain wavelength. These very specific wavelengths give the technique excellent specificity and detection limits in the AAS analysis. Absorption for each element is specific, no other elements absorb this wavelength. Typical applications of AAS include –
- Quantitative metal concentrations in solution
- Analysis of lead in paint
- Monitoring of trace metals in industrial effluent streams
- Trace elements in product/raw materials along with ICP-MS
- Analysis of additives and purity in steels and other metal alloys
- Analysis of low-level contaminants
Several analytical techniques have been applied for detection and quantitative estimation of trace metals in different types of matrices. Classical techniques based on gravimetric and titrimetry provided good accuracy but were time-consuming.
Increasing demand for high speed analysis led to the introduction of instrumental methods such as Ion selective electrodes, UV-VIS spectroscopic techniques, Atomic Absorption Spectroscopy, ICP – OES and ICP – MS. The choice of technique depends on the required detection levels, available sample quantity and most important available budget. The topic is covered to some extent in the article which elemental analysis technique is right for me.
Atomic Absorption Spectroscopy is a moderately priced instrumental analysis technique which provides a high degree of accuracy and precision of results. Due to its high analysis throughput, it finds its rightful place in university laboratories, pollution control laboratories and industrial quality control laboratories.
The present article highlights some areas where an awareness of working with an Atomic Absorption Spectrometer will prove to be an asset in enhancing your professional growth.
In case you are engaged in any of the activities or areas discussed in the article or wish to land into such areas, you will stand to gain through up-gradation of your knowledge and technical skills on this technique.
Atomic Absorption Spectroscopy Applications
Mining and Geology – The elemental composition of minerals and rocks provide valuable information on the commercial feasibility of conducting mining activities in areas explored. After mining, the ores and minerals need to be tested for composition for the efficiency of refining operations. Similarly, trace metal analysis is of great value in prospecting for oil and water deposits.
Gemstones are also graded on the basis of the presence of certain trace metals. Elemental composition of archaeological artefacts is helpful for tracing their source.
Environmental Monitoring – Environmental monitoring for trace metal contamination of industrial effluents, oceans, rivers and lakes is important for establishing the safety of water for drinking and commercial use. It is important to establish if such samples are within the safety limits set by regulatory bodies. Environmental monitoring also plays a significant role in the evaluation and feasibility of the site for setting up commercial projects.
Materials Development – Common properties of materials such as hardness, brittleness, grain size, crystallinity and amorphous nature are significantly influenced by composition and trace metals. Trace metal analysis can provide useful information on the performance properties of such materials.
Pharmaceuticals – Trace metal analysis plays an important role in formulation development, catalyst efficiency and dosage limits. Most elements have a beneficial role up to certain prescribed limits but beyond such limits the effects are harmful.
Foods and Beverages – In synthetic processed foods, metal pickup takes place due to contact with processing equipment and catalytic conversions. Consumer awareness on food safety is increasing by the day so manufacturers have to ensure that the trace metals do not exceed the permissible limits and this requires rigorous quality control through atomic absorption spectroscopy and other sophisticated instruments.
Oil and Petroleum – Both edible oils and mineral oils require refining before consumption. Such refining operation can involve distillation as well as catalytic refining. Uptake of metals during such operations can lead to deterioration of performance or consumer hazards. Trace metal analysis of engine oil provides useful diagnostic information on the wear and tear of engine parts.
Agriculture – Trace metal constitution of soils in addition to their acidic or basic nature is essential to establish their productivity and nutrient value. Trace metal composition of plants (leaves, stems and roots) gives a fair idea on how the uptake of minerals gets distributed under different growth conditions
Forensics – Trace metal analysis provides valuable information on specimens such as stomach contents for food poisoning, paint chips, fibres and hair strands collected from the scene of a crime.
Types of Atomic Absorption Spectroscopy
Nowadays, Atomic Absorption Spectrometry (AAS) systems are comparatively inexpensive instruments. Some also predict multi(few)-element capability. There are various types of AAS – Flame (F AAS), Cold vapour (CV AAS), Hydride-generating (HG AAS), and Graphite furnace (GF-AAS) systems.
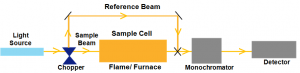
Instrumentation of AAS
Atomizer
The sample must be atomized first in order to be studied. Atomization is an important step in AAS as aids in determining the sensitivity of the reading. An effective atomizer creates a large number of homogenous free atoms. Though there are many types of atomizers present, only two are commonly used: Flame and Electrothermal atomizers.
Radiation Source
There is a radiation source which irradiates the atomized sample. The sample absorbs some of the radiation, and the rest passes through the spectrometer to a detector. Radiation sources are of two categories: Line sources and Continuum sources. Line sources excite the analyte and thus emit its own line spectrum. Continuum sources have radiation that spreads out over a wider range of wavelengths.
Spectrometer
Spectrometers are used to differentiate between various types of wavelengths of light before they pass to the detector. The spectrometer in AAS can be either single-beam or double-beam.
Single-beam spectrometers require radiation to pass directly through the atomized sample. Whereas, double-beam spectrometers require two beams of light – one beam that passes directly through the sample, and another that does not pass through the sample at all.
Learning Atomic Absorption Spectroscopy
Understanding the basics and operation of Atomic Absorption Spectroscopy is a career objective of every trace metal analyst. Today’s trace metal analyst cannot afford to remain ignorant of this well-established technique.
Awareness and need for testing of materials existed even in ancient times and has grown to keep its pace with the growth of human civilization. Today you cannot imagine any man-made product be it a machine tool, glass decorative item, food product, pharmaceutical, plastic ware or for that matter any other product which has not undergone quality control using analytical techniques at some stage of its manufacture.
Even our natural resources such as water, air, food grains, fruits and vegetables are certified for human consumption after undergoing laboratory testing.
Analysis of trace metals gained significance on the onset of the age of metals. Even in those times, it was common knowledge that composition of alloys has a bearing on properties of metals to be used for the development of weapons for warfare, hunting, implements, storage of foods and drinking water.
Efficacy of herbal medicines based on ancient systems such as Ayurveda, Unani and Siddha is dependent on the presence of trace metals or their oxides incorrect amounts. An excess of such components could be disastrous to the consumer.
Knowledge of AAS, its potential applications and operational aspects is an asset for any analytical scientist. The Certificate course on Atomic Absorption Spectroscopy is designed keeping the requirements of the working chemist in mind.
The AAS programme at Lab Training is designed to provide an insight into the basics, operations and maintenance exposure to ensure a trouble-free operation of the system.
The learners get the additional benefits of understanding the workplace environment through interaction with our technical experts. The course also lays stress on basic laboratory procedures which often get overlooked in the university curriculum.
The programme is beneficial to fresh graduates who are looking forward to a career in industrial quality control and research labs and also to the working professional who gets an opportunity to upgrade their skills and awareness of the advances in the technique.
The programme is interactive in nature with quiz sessions between different modules. On completion of the programme, a certificate of participation is awarded and placement assistance and guidance are provided to desirous participants.
Glossary of AAS terms
The glossary will help you to understand the terminology in case you aren’t already familiar with the technique.
| Atomic Absorption Spectroscopy | Study of element specific light absorption by ground state atoms for estimation of concentration of the element in the sample solution. |
|---|---|
| Atomisation | Process of reduction of sample to ground state atoms by application of heat by means of a flame or a graphite furnace. |
| Atom | The smallest particle of an element or compound. It comprises of a central nucleus containing neutral particles called neutrons and positively charged protons. The electrons revolve the central nucleus in shells of different energy levels. The number of electrons equals the number of protons in the neutral atom. |
| Atomic Emission Spectroscopy | Qualitative identification and quantification of element by emission of characteristic wavelength of light on excitation of an element by means of a flame or plasma |
| Atomic Fluorescence Spectroscopy | Measurement of light emitted on decay of elements from excited states.Measurement is made at an angle to the optical beam path so that the detector sees only the fluorescence in the flame and not incidental light from the lamp. |
| Absorbance | The amount or fraction of incident light absorbed by the ground state atoms. It is directly proportional to the number of ground state atoms in the beam path and also on the optical path length of the flame in accordance to Beer Lambert law of light absorption |
| Absorbance unit | a ratio of intensity of transmitted flight to the intensity of incident light. It is a unit less quantity but is commonly expressed in absorbance units (EU) |
| Aspiration | losses of reduction of liquid sample stream into fine droplets for introduction into the flame |
| Acetylene | Commonly used gas as fuel to support combustion of the flame.Provides temperatures in the range 2150-23000C |
| Argon | Gas used commonly as a filling gas in hollow cathode lamps and as sample carrier in graphite furnace analysis |
| Air | Used as oxidant in combination with acetylene as fuel gas to support the flame |
| Air compressor | Device for delivery of air to the atomic absorption spectrometer. Oil less air compressor is preferred as contamination from oil is thereby avoided |
| Burner | A component of AAS system made of solid metal body having slit on the flat top surface to provide the flame required for atomisation of the sample |
| Blaze angle | It is the angle of cut of a mechanically ruled grating at which the angle of incidence is equal to the angle of reflection so that light intensity is greatest with minimal loss due to diffraction. For greater efficiency dual blazed ratings are used which provide greater light throughput over the wavelength range of the spectrometer |
| Background | any extraneous light other than the transmitted light that reaches the detector and affects the signal absorption |
| Background correction | Means applied to reduce the effects of background on the signal |
| Concentration | The amount of element present in a unit volume of solution.Usually expressed as ppm (mg/lit) or ppb (μg/lit) |
| Characteristic concentration | Concentration of an element expressed in mg/lit required to produce a 1% absorbance or 0.004 absorbance signal. Knowledge of characteristic concentration helps predict the concentration range required to produce optimum absorbance levels for analysis |
| Collimation | Condensation of beam of light as per size requirement |
| Cathode | An electrode inside the lamp made from the pure metal whose analysis is required in the sample solution |
| Chopper | A half transparent half opaque disc that rotates in the beam path to split the beam so as to alternately allow its passing through the sample or around it to give effective double beam performance |
| Cold vapour mercury analyser | Analyser fo mercury without using a heated sample cell as mercury is only element which exists as a liquid at room temperature |
| Deuterium sources | A broadband light source for providing background correction in flame analysis |
| Detector | A component of the system that records the intensity of the transmitted light. Photomultiplier tube is the commonly used detector in AAS |
| Double beam system | Optical arrangement which alternately permits the light beam to pass through the sample and round it as a reference beam. |
| Desolvation | Refrom sample droplets by heat inside the flame |
| Exhaust ventilation system | An assembly for removal of hot corrosive combustion gases and vapours arising from the flame |
| Electrode/discharge lamp | a lamp used for analysis of volatile elements.It is a high energy light source which has a longer life than corresponding hollow cathode lamps. |
| Excitation | Excitation of a ground state atom to higher energy states by means of electromagnetic radiation |
| End Cap | Removable cover of spray chamber that serves to introduce sample into spray chamber and also hold the nebuliser |
| Flow spoiler | A device inside spray chamber used for removal of large droplets of sample |
| Flame | Atomisation system which uses a flame. Commonly air – acetylene gas mixture or nitrous oxide – acetylene for higher temperature combustion |
| Flashback | Reverse movement of flame inside burner towards spray chamber due to greater proportion of oxidant or even pure oxygen in flame. It often results to a loud explosion and damage to spray chamber |
| Furnace | A graphite tube about a cm long with a hole on top for atomisation of sample using electrical heating of the tube |
| FIAS | Flow injection analysis system for automated analysis using hydride generation |
| Graphite furnace | same as furnace |
| Grating | A light dispersing device used in the monochromator |
| Hollow cathode lamp | A light source used for AAS analysis which is specific for metal to be analysed in the sample. For some elements multielement hollow cathode camps are also used |
| Hydride generation technique | Used for analysis of volatile hydride forming elements such as As, Bi, Ge, Pb, Sb, Se, Sn, Te. |
| Impact bead | A device inside spray chamber for removal of large sized droplets from sample stream |
| Interference | Effects resulting in variation of results due to spectral or non-spectral interferences |
| L’vov platform | small platform made from solid pyrolytically coated graphite which is placed at bottom inside graphite tube. Sample is put into a depression in the platform. Permits uniform heating and delays atomisation till stable temperature conditions are preached inside the furnace |
| Monochromator | A device used for dispersion of incident light using prism or grating, reflecting mirrors and a combination of entrance and exit slits for isolation of required wavelength and collimation of the light beam |
| Mirror’s | Light reflecting component of monochromator with a aluminium or gold coated surface to reduce corrosion damage and provide high reflectivity |
| MHS | Mercury hydride system for analysis of volatile elements by hydride formation |
| Matrix interference | Interference arising due to differences in parameters such as viscosity, surface tension between sample and standards solutions |
| Microwave Reaction System | Automated digestion of samples in closed tubes using sonic waves. It offers advantages of speed of f digestion, cost and freedom from toxic vapours |
| Matrix modifier | Substance used for reduction of chemical interferences |
| Nebuliser | A device for producing an aerosol of sample inside spray chamber |
| Orifice | Small bore tube opening |
| Polychromatic | A light dispersion device using an array of detectors for simultaneous detection of elements in a sample |
| Photomultiplier tube detector | A detection device used in AAS which amplifies the current produced by impact of photons on a light sensitive surface |
| Prism | A light dispersing element |
| Quartz | A UV transparent material used for making hollow cathode lamp and graphite tube end windows |
| Slit width | Width of monochromator entry and exit slits expressed in millimeters |
| STPF | Stabilised temperature platform furnace is a combination of instrumental and analysis factors for providing high accuracy of results |
| Transverse heating | Heating of graphite furnace perpendicular to its axis to provide uniform heating of graphite tube along its length |
| UV range | Wavelength range 180 – 350 nm. Most elements have specific absorption bands in this region |
| Zeeman background correction | Advanced background correction used in graphite furnace analysis involving application of a magnetic field perpendicular to the graphite furnace. Effective for background correction of complex matrices. |
Refresh your concepts by registering for the free course which will provide you an introduction to the technique and even prepare you for an interview if you are applying for a job in the laboratory equipped with AAS systems.
Sign Up Now!
Want to read all the AAS free course modules right now? Here are all links to all the modules for you!
- Introduction to Atomic Absorption Spectroscopy course
- Module 1 : Scope of Spectroscopic Analysis
- Module 2 : Evolution of Atomic Absorption Spectroscopy
- Module 3 : Introduction to AAS component parts
- Module 4 : Types of Light Sources in AAS
- Module 5 : Flame Atomic Absorption Spectroscopy
- Module 6 : Graphite Furnace Atomic Absorption Spectroscopy
- Module 7 : Dispersion and Resolution of Light in Atomic Absorption Spectroscopy
- Module 8 : Interferences in Atomic Absorption Spectroscopy
- Module 9 : Background correction in Atomic Absorption Spectroscopy
- Module 10 : 10 Interview questions in Atomic Absorption Spectroscopy
Want to learn more about Atomic Absorption Spectroscopy? Continue with our library of articles on AAS below –
We regularly publish articles specially to help you upgrade your laboratory skills and to expose you to new concepts and developments in the field of Atomic Absorption Spectroscopy.
You’ll find the list is ever growing with inclusion of newer published articles. We are confident that you’ll find the article content of immense use. Continue learning more about Atomic Absorption Spectroscopy by clicking any of the articles that interest you.

[…] advanced and most commonly used sensitivity sampling technology. The primary aim of this furnace in AAS deals with high solid samples and slurries, which can be analyzed, along with soluble samples. This […]