Gas Purification Requirements in Gas Chromatography
Gas chromatography uses mainly three types of gases:
- Carrier gases: nitrogen, hydrogen or helium
- Fuel: hydrogen
- Oxidant: zero air
Gases used for gas chromatography analysis should conform to specified purity requirements. Most laboratories use gas cylinders or gas generators from reputed sources. However, it is advisable to further purify the gases before use on gas chromatography. Before talking about purification let us discuss briefly purity and other general requirements of gases used for gas chromatographic analysis.

Carrier gas
Carrier gas should contain less than 1 ppm of oxygen, moisture or other contaminants. Helium and nitrogen are commonly used. Hydrogen is an ideal choice but is very rarely used due to its high flammability hazards. Stainless steel or copper tubing free from grease or organic matter is used. Carrier gas should be filtered using moisture trap, hydrocarbon trap and oxygen filter.
Hydrogen
Hydrogen is commercially used as a fuel to support combustion in Flame Ionization detection. Purity of hydrogen should be at least 99.995% and it should be passed through a moisture filter
Zero air
Zero air plays the role of oxidant to support combustion. It should contain less than 0.1 ppm of total hydrocarbons. As for carrier gas stainless steel or copper tubing can be used. It needs to be free from moisture so moisture trap should be used in its flow path
Tubing requirements
Copper and stainless steel tubes are generally used. Plastic, Tygon or PVC tubing should be avoided as water and air can permeate through such materials. Plastics also give off organic impurities which can result in baseline instabilities and extraneous peaks. Length of tubing should be kept to minimum to avoid excessive pressure drop and number of bends in lines should also be kept to the minimum.
Filters and traps
Traps help remove moisture, oxygen, hydrocarbons and other impurities from gas lines. Metal or glass traps (self indicating) are commonly used. Plastic traps like plastic tubes are not recommended. Traps are available with standard 1/4”or 1/8” compression fittings.
Moisture traps are generally self indicating type and packed with molecular sieves or silica gel which will reduce both oxygen and moisture to less than 15 ppb.
Hydrocarbon traps are useful for removing hydrocarbon impurities by absorption on activated charcoal. A 20 μm frit removes particulate impurities. Indicating hydrocarbon traps are used for removal of oil contamination from oil lubricated air compressors for FID operation.
Oxygen traps remove oxygen down to 0.1 ppm.Oxygen contamination can produce excessive column bleed at high temperatures. Care needs to be exercised in handling oxygen traps as these are packed with highly reactive material whose exposure should be avoided.
Installation sequence of traps in carrier gas stream
A proper sequence needs to be followed for installation of traps in carrier gas stream.
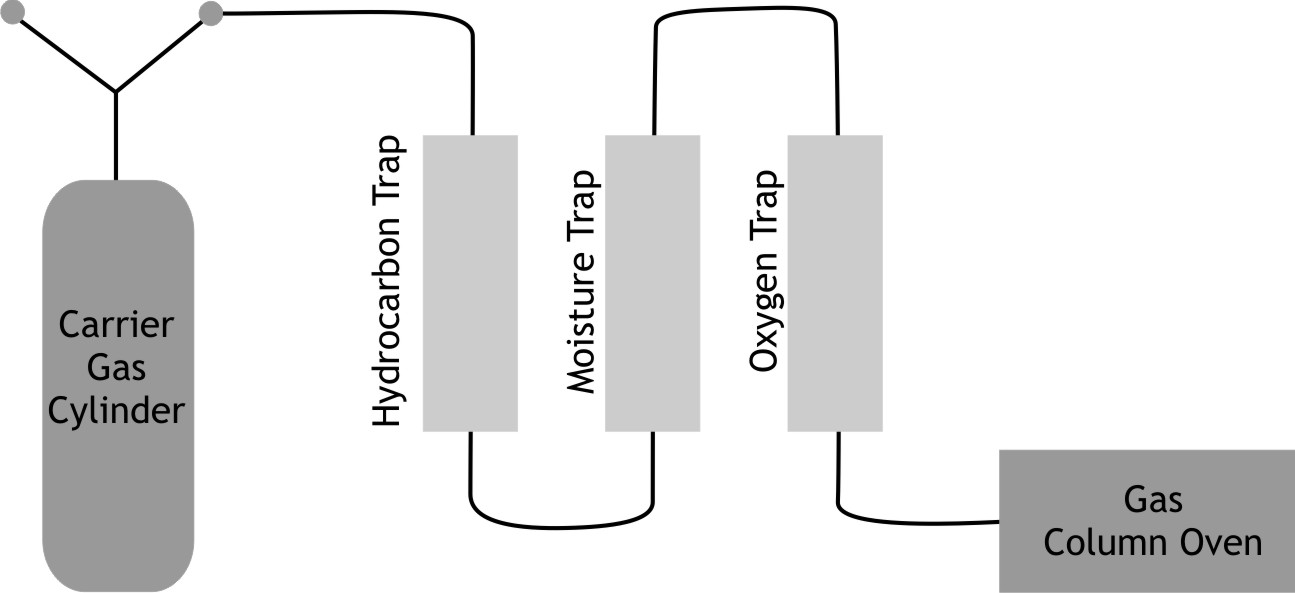
First in line from gas tank is hydrocarbon trap. This prevents contamination of molecular sieve in moisture trap by hydrocarbons. Second is moisture trap and third is oxygen trap which is as close to gas chromatography oven as possible. This will help eliminate oxygen that may enter from leaking fittings downstream.
It is advisable to mount traps vertically to prevent channeling.
Gas purification of commercially available gas supplies is necessary to generate stable baselines without noise or ghost peaks in gas chromatographic analysis.
Please share your experiences and comments with us.



How Describe G C Graph
Hello Krishna,
Please go through the article “How to read a chromatogram”. Hope you will get your answers.
what is the reason keeping hydrogen 40 and oxygen 400 in FID
Hi, This is based on simple stociometry ie. how much Hydrogen will need how much Oxygen to completely react and form water.
Dear Dr. Deepak Bhanot,
I am GMP inspector and I would like to share with you my experience with you about the quality of the carriers used for GC.
During my inspections, I rarely see a QC lab testing the carriers. It is very hard to explain to the companies the possible impact of the quality of the carriers on the quality of the analytical results. I had several occasions to talk about it with GC experts, all of them agree with me; however, I cannot find any scientific published article nor an official reference about it to support the relevance of the carrier test. Could you kindly give me any suggestion, please?
Thanks a lot
Anna
Hi Anna
Your concern on purity of carrier gas is appreciated.Please find links of three articles which were published earlier. Hope you will find the information useful.
https://lab-training.com/2016/11/15/recommendations-switching-gas-cylinders-laboratory-gas-generators/
https://lab-training.com/2014/04/04/hydrogen-helium-or-nitrogen-which-is-most-suitable-as-a-carrier-gas/
https://lab-training.com/2013/11/19/gas-purification-requirements-in-gas-chromatography/
thanks a lot for the prompt reply. I have already read the articles proposed by you. There is nothing about the effects of contaminants, for example. For companies the suitability test is enough to justify the quality of the carrier. Any suggestion?
Thanks a lot.
Kind regards,
Anna