Why are HPLC columns shorter than GC columns?
HPLC and GC are separation techniques which have gained a strong foothold in chemical laboratories. The basic purpose of both techniques is to separate and quantify the components of organic mixtures.
Have you ever wondered why HPLC columns are shorter and broader than GC columns. Before you answer this question you should understand the benefits of long column lengths. As the column length increases the interaction time between the eluting compounds and stationary phase increases thereby increasing column efficiency and resolution. This results in well separated peaks. However, the column length cannot be increased indefinitely due to practical problems faced due to increased column head pressure and also increase in analysis time.
HPLC columns

The dimensional requirements of HPLC columns depend on the separation objectives. Usual analytical applications require columns of length 15 – 25 cms with 4.6 mm id whereas preparative columns can be broader with diameters ranging from 20 – 50 mm.
GC columns

GC columns are basically classified as packed columns and capillary columns. Packed columns are generally about 1.5 m or 6ft long and 1/8” or 3.2mm outer diameter.. Capillary columns on the other hand are several meters in length(10-100m). Packed columns are made of glass or stainless steel whereas capillary columns are made of fused silica flexible tubing. Flexible tubing permits accommodation of long length columns as circular coils into the column oven
Now examine the difference in the separation process in the two techniques to arrive at logical answer to the basic question on length differences between GC and HPLC columns..
Nature of mobile phase – the first and foremost reason is nature of carrier fluid termed as mobile phase. HPLC uses a liquid as a carrier of sample through the column whereas in GC a gas stream serves to carry the sample. Liquids have higher viscosity than gases and therefore encounter greater resistance during passage through the column. The longer the column greater will be the resistance to flow of mobile phase therefore HPLC requires shorter column lengths
Sample volatility – the sample injected into the HPLC is a liquid whereas in GC it can be either a liquid or a gas. However, before entering the GC column: liquids get converted to vapours due to high temperature in the injector block. A gas encounters less resistance in the GC column which permits use of longer column lengths.
Sample stability – samples analysed by HPLC are generally thermally labile so they are in liquid phase in the HPLC column at room temperatures. On the other hand samples entering the GC column are gases having lower molecular weights and boiling points. Such compounds are easily vaporized and remain as gases during passage through the column.
Further reduction in column length particularly in new UHPLC applications have resulted in accelerated analysis with improved sensitivity.Future trends in analytical applications are bound to reduce HPLC column lengths and analysis time from several minutes to a few seconds.


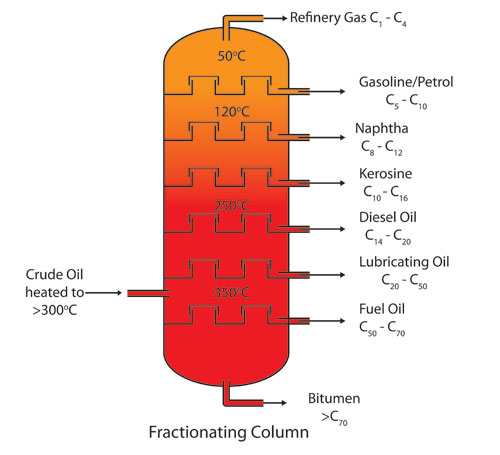
please tell me interview quation and anser hplc &gc
Hi Sanjeev,
The answers to interview questions can be found in the course material itself.These are only some specimen questions designed specially to encourage you to go through the course thoroughly and increase your inquisitiveness.
Really so good and very useful to develop own analytically skill………..theoretically..
Thanks. Nice to know you liked it
It iz very usefull and i learnt basic concepts ang qualified bps 17 lecturership
in analytical chemistry
Thanks Asma for your encouragement.
Dear Sir
I want to know where is the scope of hplc in scientific world and what types of samples(organic and inorganic) can be analysis by this magnifiicent instruments .
Dear Sandeep,
You can subscribe to the free course on HPLC which will provide you the required details and later if you feel interested you may also subscribe to the more elaborate paid certificate course .The technique holds great promise in analysis of pharmaceuticals, foods,cosmetics,environmental samples,clinical and life science applications.
dear sir,
in gc analysis some times we are observed spikes,may be suspected due to any disturbance at environment and power fluctuation. these are not merged with target peaks.so we are valid these chromatograms are valid with comment.
is it correct practice or not can you explain me.
I hope these spikes are random and you do not get them on repeat injections. In case these are random you may ignore them.
Dear sir,
How can i phrase the answer for a proper write?
Hi Jay,
The article has provided you relevant information. You can summarize to write s short write up
Sir I want to know about the time of course that certificate will be carying.
The course has no time limit. You can complete it at your own pace and convenience. However,our registrants normally take 2-3 months to complete the course and answer the evaluation sessions
Dear Dr. Bhanot,
Thank you for the knowledge you always give to us. Please can I use ZR-PS column for histamine and other biogenic amines analysis? Which mobile phase will be appropriate. Thank you.
Dear Meinster,
Offhand it is difficult to recommend a column or mobile phase for a specifcic application. The choice of column will depend on your sample matrix, concentration level, etc though there are some references where Agilent Zorbaax DB columns have been used.Ideally you should do some literature survey to decide on your choice which would be meeting your analysis requirements. You may also narrow down on equivalent columns that are available in your laboratory.
Regards
It iz very usefull and i learnt basic concepts of HPLC and GC . Thanking you sir ! ☺️
Thanks Rupesh for the encouragement. It is a great feeling to know that the information has proved useful to you.
Very good. I learnt a lot from it. Please how can I enroll for certificate courses.