Concept of Theoretical Plates in Column Chromatography
As a chromatographer you would be interested in getting the maximum return from your column in terms of performance efficiency. Useful tips on care of HPLC columns have been suggested earlier to preserve the performance features and improve useful life of your column. Now the question arises as to how do you quantify efficiency and decide if you can still use your column to get reliable separations. The answer lies in keeping a track on theoretical plates over the lifespan of a column.
The setup of chromatography consists of two phases: the mobile phase and stationary phase. The prior one is a fluid in which the mixture that has to be separated gets dissolved. After this, the mixture containing fluid is passed through the stationary phase where the separation happens. This is where a column comes into the role.
It contains the stationary phase and lets the mobile one pass through it. That brings us to the concept of theoretical plates in column chromatography. Let’s understand what it is before moving on to its importance.
What Are Theoretical Plates in HPLC?
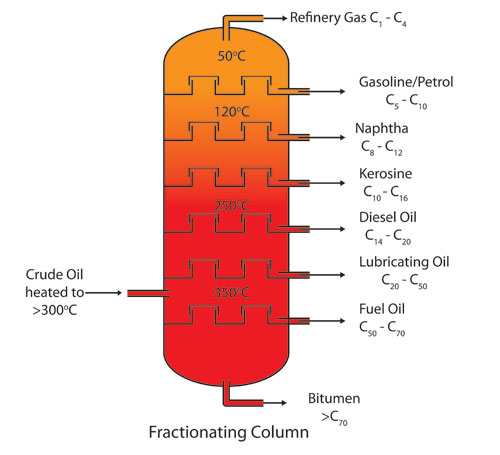
A chromatographic column does not consist of any physical plates which can be measured from time to time to rate a column’s performance efficiency. It is only a theoretical concept drawing a similarity to the huge fractional distillation columns used in the refining of crude petroleum.
Such distillation column actually comprises separator plates which help separation and isolation of different petroleum fractions which are collected as separate streams. Now, let’s see from where this concept came into existence.
History of Theoretical Plates in HPLC
Earlier, the process of fractional distillation used to have different lengths and designs of columns. So there was a need for separation efficiency accounting in that, which brought in the concept of theoretical plates. The basis of this plate theory of chromatography was the assumption that the procedure of distillation took place in various stages along the used column’s length.
However, the point to be noticed here is that the fractional distillation does not come under the category of chromatographic processes.
Why Are These Plates Important?
Theoretical plates in column chromatography are directly related to the efficiency of its column. Therefore, it can assist in improving the resolution, which is directly proportional to the square root of theoretical plates’ number when all other variables are kept constant. This means the resolution will increase by a factor of two each time the number of plates is raised by four.
From all these things, it is clear that the more theoretical plates imply the better quality of separations as more equilibriums can be attained between stationary and mobile phases.
You can understand this with a simple example of stairs. As the number of stairs reduces, the length of each stair increases. So you need to stretch your legs more to climb every stair. On the other hand, shorter stairs will definitely increase their number but they will reduce your efforts of climbing each one of them. The same thing goes with theoretical plates. More plates make the overall separation process simpler.
These factors show that theoretical plates are important for increasing the efficiency of the column and making the chromatographic process more simplistic.
The Number of Theoretical Plates in ChromatographyIt must be clear by now that no such plates exist in reality in the chromatographic columns. You just have to imagine that your column is divided into a number of sections or plates. As stated earlier, a sample component spends a finite time in each plate as it moves the column length and this is the time required to establish equilibrium over the length of the plate between its concentration in the stationary phase and the mobile phase.
In simple terms, it can be stated that a theoretical plate represents the distance that is needed for every adsorption-desorption step. The movement of the analyte is assumed from plate to plate as a series of equilibrated mobile phase plugs. In other words, the greater the number of such plates, the more efficient is the separation power of the column.
The number of plates (N) over the column length (L) is dependent on the plate height (H)
N = L/H
Smaller plate height implies large number of plates in the column and higher is the column efficiency. H is also referred to as height equivalent to a theoretical plate (HETP) and smaller the value of HETP the greater is the column efficiency.
We can also notice that the column length directly affects the number of plates. It means the longer the column, the more plates can be incorporated in it. Therefore, you can achieve better efficiency by opting for columns that are lengthier.
Calculation of the number (N)
N is calculated from the peak of the injected standard under prescribed operational conditions
N = 16\((\frac{t_r}{W_b})^2\)
where TR is the retention time of the peak and Wb is the peak width. In case peak width is measured at half height or W1/2 then the relation is expressed as
N = 5.54\((\frac{t_r}{W_1_/_2})^2\)
In above calculations retention time and width should be expressed in the same units
Present day chromatographic softwares provide number N without the need for manual calculations but the basic assumption is that the peak shape should be Gaussian.
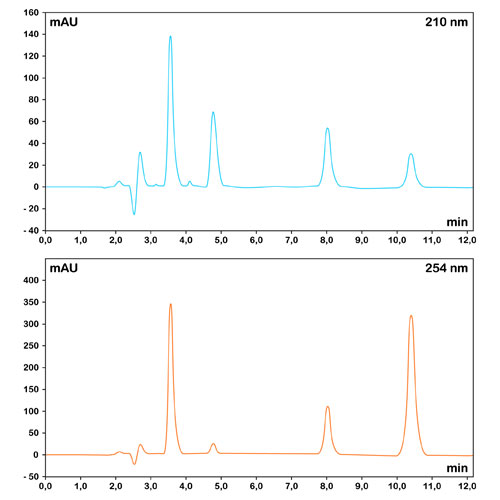
The number of theoretical plates is dependent on several operational parameters such as flow rate, analyte characteristics, temperature and sample size. Overloading a column rapidly deteriorates its performance.
The Height of Theoretical Plates in Chromatography
Another factor that can be used to measure the column efficiency is the height equivalent to a theoretical plate (HETP). Martin and Synge also referred to this factor as the plate’s thickness.
This is the same height that was used in the formula of the number of plates. It is generally calculated in millimeters. From the stairs example, we can see that the height of each plate determines the number of plates and the efficiency of the column. You can also see the formula:
HETP = L/N, where L is the column’s length and N is the number of plates.
So the shorter we keep the height, the better efficiency can be obtained because of the reduced depth of the stationary phase.
Effects on Plate Number
Here are a few factors that impose strong to medium effect on the number of theoretical plates:
- Size of the particle (very strong impact)
- Inter-connecting tubing’s dead volume (very strong impact)
- Profile of gradient (strong impact)
- Temperature of column (strong impact)
- Length of column (strong impact)
- Composition of stationary phase (medium impact)
- Composition of mobile phase (medium impact)
- Shape of the particle (medium impact)
- Uniformity of the particle’s shape (medium impact)
Hope all these things made the plate theory of chromatography clear to you.




Very good lecture…. Easy language and easy concept transferring way..
Yes I understood very clear
Thanks