What is Chromatography?
If you work in the chemical industry, you must have heard about the technique of chromatography. Despite being a common procedure, many people still aren’t familiar with what is chromatography and how exactly it works. If you are also confused about the topic, you have come to the right place. This article includes all the details about chromatography. Let’s get started with the meaning and definition of chromatography.
Meaning of Chromatography
As per dictionary definition Chromatography is a method for separating the constituents of a solution(gas or liquid) by exploiting the different properties of different molecules. The technique employs a mobile phase(gas or liquid)to transport the solution to be analyzed through the stationary phase (solid or liquid)which absorbs or impedes different components of the solution to different degrees and thus causes their separation as different layers. It is an invaluable tool in the hands of analytical scientists for the separation and quantification of components in a mixture of organic compounds.
History and Basics of Chromatography
The technique had its origins in the pioneering work of Mikhail Tswett who separated plant pigments in 1900 using a packed glass column. As the separated pigments were differently coloured and separated as distinct bands the technique was coined as chromatography meaning separation by colours.
Over the years chromatography has evolved and advanced into versions such as HPLC, GC, HPTLC and SFC all of which to this day are based on the separation of mixture components through selective physico-chemical interactions and partitioning between the stationary and mobile phases.
Why Do We Use Chromatography?
Since its evolution chromatography has found wide use in separation of components of mixtures ranging from simplest gases to most complex hydrocarbon mixtures containing hundreds of different compounds. Samples can be gaseous, liquids or even solids which are readily soluble in suitable solvents.
Due to its versatility the applications cover complete range of chemical compounds having diverse characteristics such as boiling range, molecular weights, volatilities, and thermal stabilities. Gas chromatography, Liquid chromatography, Thin-layer chromatography, Super critical fluid chromatography and hyphenated techniques such as GC – MS, and LC – MS cover a vast number of applications in diverse areas such as pharmaceuticals, material development, foods, petroleum products and forensic investigations requiring very high resolution and detection limits.
A few application of chromatography in different industries are:
Chemical Industry
- Contaminants’ detection in pesticides and oils
- Water sample testing
- Air quality checks
- Numerous applications in life sciences
Pharmaceutical Industry
- Taking element composition and its molecular weight to separate compounds
- During the process of drug development
- Detecting chemicals or trace elements in various samples with proper analysis
- Examination of mixture purity
- Identification of unknown compounds
Forensic Industry
- Blood and hair sample analysis under crime scene testing
- Forensic pathology
Food Industry
- Identifying the food products’ nutritional values and quality
- Detection of additives and food spoilage
Molecular Biology Studies
- The fuel industry, biochemical processes, and biotechnology use HPLC for purification and fractionation process
- Metabolomics and proteomics study through specific chromatography techniques
- Nucleic acid research with specific chromatography methods
These are only the popular applications of chromatography. The technique is used in various other places and industries.
Basic Working of Chromatography
The exact process of chromatography will depend on where you want to use it and which method you opt for (these will be explored later in the article). However, we can still define a basic procedure that consists of simple four steps:
- Step 1: Careful exposure of a specific analyte amount to the mobile phase stream that was already running.
- Step 2: Then, the analyte is carried out through the stationary phase with the help of the mobile phase.
- Step 3: Finally, the separation takes place when the analyte components react with the stationary phase at different levels. Some of them react more, while others don’t.
- Step 4: The separated out analyte components are then again carried out by the mobile phase. It then takes the components to a separate instrument. There, they get quantified with the proper detection of their presence.
And in these four steps, the process gets complete. We can say that chromatography is divided into three basic components: Mobile Phase, Stationary Phase, and Separated Molecules.
Let’s now get into the details of the use of different parts of chromatography.
Chromatography Parts
The four steps aren’t enough to understand how chromatography works. You also need to know about the different elements of the process, along with their working. Only then will you be able to learn the concept.
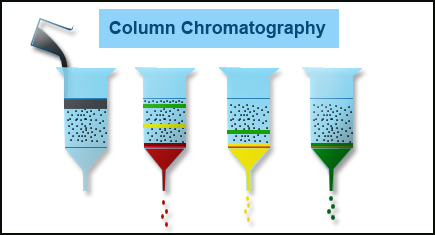
Chromatography Columns
Separation of sample components before detection is the essence of chromatographic techniques. A chromatographic system uses a column to achieve the desired separation. A column comprises of a tube packed with a stationary phase on which separation takes place based on physico – chemical interactions of separating compounds with the stationary phase. The mobile phase or the carrier gas elutes less weakly retained components first followed by more strongly retained components.The column dimensions and composition is based on the chromatographic technique selected and the degree of required separation. In general HPLC columns are shorter and wider than GC columns.
Chromatographic Detectors
After separation the individual components reach the detector which provides response in terms of the area or peak height depending on the amount of the eluting compound. Each chromatographic separation technique offers a range of detectors depending on the nature of eluting compounds.
Detection can be specific for a particular compound or a range of compounds or it can depend on some physical properties such as reflective index of the mobile phase. Such detectors are referred to as bulk property detectors in comparison to selective or specific detectors.
Chromatographic Data
Chromatographic separations appear as peaks in the chromatogram except for thin-layer chromatography for which separate zones are seen on the plate. Chromatographic peaks are separated in time in the chromatogram depending on the nature of separating compounds and the separation efficiency of the column. Sharp well resolved peaks indicate high degree of column resolution whereas broad or overlapping peaks are indicative of poor resolution.
Each peak represents a sample component and is characterized by its retention time under defined operating conditions. For purposes of quantification the peak height or more accurately the area under the peak defines the amount of the component present in the mixture.
Quantitation methods require percentage composition of a particular component in the mixture and such estimations are made on the presumption that the detector responds to all the components equally and peaks are generated for each compound.
%of analyte = \(\frac{Area of analyte peak}{sum total area of all peaks}\)X100
Quite often the amount of analyte is specified in relation to another compound which is referred to as a reference standard having established traceability. Such compounds should be available in pure form and have chemical identity which is same or close to the identity of the analyte to be determined.
Modern-day analytical systems come equipped with sophisticated application softwares which are capable of operational control, data interpolation and calculations to achieve the desired results.
Common Chromatography Methods
A few general chromatography methods that are divided based on the separation basis of the elements are:
- Column Chromatography
- Affinity Chromatography
- Thin-Layer Chromatography
- Ion-Exchange Chromatography
- Gas Chromatography
- High-Pressure Liquid Chromatography (HPLC)
- Paper Chromatography
- Gel-Permeation Chromatography (Molecular Sieve)
- Dye-Ligand Chromatography
- Pseudo-Affinity Chromatography
- Hydrophobic Interaction Chromatography
Now you have the answer to the basic question ‘What is chromatography?’you will be eager to know more about the chromatographic techniques .Simply register to our free courses and advanced certificate programmes listed under courses on the main menu.



Dear Sir,
I am woking with a drug laboratory now and I am learning a very good concepts with your lab training programme.But I wok with an AAS in our laboratory.But I don’t understand the standard addition techniques in AAS.
So, if you kindly give the proper concept of standard addition techniques in AAS instrument with an example elaborately I shall be grateful to you.
Thanking You, Your’s faithfully,
Date-16.9.2013 Asit Kumar De
Dear Asit,
I believe that the concept is universal irrespective of the analytical technique.The only difference is that in case of chromatographic analysis it refers to adddition of the organic compound whereas in AAS it refers to addition of the same element in known concentration whose composition matches the sample composition.
Hello Sir
Thanks a lot for ur update and most knowledgeable information regarding HPLC .M working in a petroleum compny I want to know whats application and parameter of HPLC AND GC for base oil SN 150,SN300 PLZ update me.
THANKS
SHAHID
Dear Shahid,
Glad to note that you found the article useful and informative.Chromatographc techniques are useful in analysis of base oils. In general there are two main applications.Firstly for studies on boiling range distribution(Ref ASTN D7500-12) and secondly for determination of Total Polyaromatic Content.A useful link is being provided on a handbook on base oils http://www.engnetglobal.com/documents/pdfcatalog/NYN001_200412073535_Base%20oil%20handbookENG.pdf
Hope you find the response useful for your applications.
Regards
Hello, I have the articles have been useful. I have been testing with capillary chromatography and electrophoresis. Basically I am using the device as LC(pressure over time) with added separation factors of the electricity. It seems to me that the sample preparation method has everything to do with accuracy and repeatability of results. My question: how does one create reference standards using the compounds? I have several phenol compounds(8-20) in solution using ethanol as liquid phase. I think I need to separate them as best I can, create two identical solutions and spike one with a reference, then test. Best regards, Desmo.
Hi Desmo,
Yes you are right-sample preparation is the key to success of any analysis.As your application is specific it would be best for you to refer to literature or search the references of standard methods for getting best results for your separations.
Dear Sir,
I had learnt a lot from your lab training module. Plz send me the basics of Method Development for any compound/molecule/biomolecule to develop a method. And also plz give me suggestions on regarding pKa of molecule.
Thanks & Regards
Mohan Thippeswamy
Thank you Mohan, we are working on some articles on method development and pKa and will be sharing them soon.