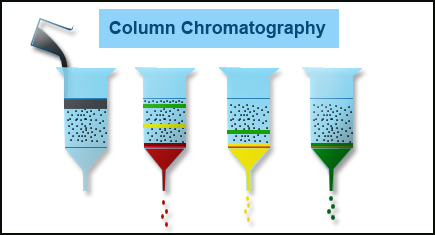
Partition Chromatography – Principle, Procedure, Applications & Types
Partition chromatography is one of the types of chromatography, introduced in the 1940s by Richard Laurence Millington Synge and Archer Martin.
Partition Chromatography Definition
Partition chromatography definition states that it is a technique mainly used for the separation of the components present in the mixture into two liquid phases that are the original solvent and the solvent coating utilized in the column.
Partition and adsorption chromatography
Adsorption chromatography is another type of chromatography where the solute (gas or liquid) molecules get directly adsorbed onto the surface of the stationary phase.
Partition and adsorption chromatography: Similarities
- Both are types of chromatography that function under the same principle
- Both are used for the separation of mixtures of compounds
- Both can separate components in all three stages namely gas, liquid and solid.
Partition and adsorption chromatography: Dissimilarities
| Partition Chromatography | Adsorption Chromatography |
| Separation is based on partition | Separation is based on adsorption |
| Liquid-solid extraction | Liquid-liquid extraction |
| Stationary phase: Liquid state | Stationary phase: Solid-state |
Partition Chromatography Principle
In partition chromatography, the separation of the components from the sample takes place through the process of partition the components between two phases, where both the phases are present in liquid form. In this procedure, the immiscible solid surface that is covered with the liquid surface on the stationary phase is in the mobile phase.
The stationary phase immobilizes the liquid surface which ultimately changes into a stationary phase. The components are separated just after the mobile phase shifts from the stationary phase. The separation is because of the differences in partition coefficients.
Partition coefficient in chromatography
When the flow of the mobile phase has been put at a stop at any interval of time, the solute acquires an equilibrium distribution between the two phases. In each phase, the concentration is given by the partition coefficient in chromatography which is represented as K = CS/CM
Here, CS stands for Concentration of solute in the stationary phase
CM stands for Concentration of solute in the mobile phase
The units of CS and CM may be different.
In a situation when the solute is equally distributed amongst the two phases, then K = 1.
Partition Chromatography Procedure
For a simple comprehension of the partition chromatography, here we are explaining the procedure for conducting paper chromatography.
Apparatus required:
- Chromatography jar
- Capillary tube
- Stationary phase
- Mobile phase – Chloroform, methanol, acetone, or ethanol
Steps:
- Selecting an appropriate type of development – Based on solvent, mixture, paper complexity, etc. the type of development is selected. Usually ascending paper chromatography is utilized as it is a simple technique to perform and also the chromatograms are quickly acquired.
- Choosing a stationary phase – Based on pores size and quality of the analyte, filter paper (stationary phase) is chosen.
- Preparing the sample – The sample is prepared by dissolving the component in a proper solvent that is employed during the procedure of producing mobile phases.
- Spotting the sample on paper – The sample mixture is applied on the filter paper at an appropriate position.
- Developing the chromatogram – With the help of a chromatographic jar, the chromatogram development is determined by drenching the paper into the solvent or mobile phase. Through the capillary action, the mobile phase is allowed to run over the test sample.
- Paper drying and identifying the compounds – After the development of the chromatograms, the filter paper is dried with the aid of an air dryer. Paper possessing different bands of molecules can be studied in the UV cabinet. Rf values are also figured out.
Types of Partition Chromatography
- Liquid-liquid Chromatography: In this partition chromatography type, instead of an adsorption column, a sheet of adsorbent paper is utilized. Based on their differential migratory velocities, the components are divided. To make the chromatograms visible, they are stained after separation.
- Gas-liquid Chromatography: This is also called Gas-liquid partition chromatography (GLPC) or vapor-phase chromatography. In this gas-liquid partition chromatography, the separation of the sample mixture is carried by an inert gas with a tube. The stuffing of the tube is done with finely divided inert solids that are coated with non-volatile oil. According to the rate determined by both its solubility in oil and its vapor pressure, the migration of every component takes place.
Partition Chromatography Applications
- To separate and detect color mixtures including pigments
- To separate and determine proteins, lipids, alkaloids, glycosides, and other biomolecules
- To isolate and detect amino acids and carbohydrates
- To examine the purity of pharmaceuticals
- In DNA/RNA sequencing
- To determine pollutants in food products and beverages
- To isolate polar and non-polar compounds
SOURCES:



Responses