Column Chromatography – Principle, procedure, Applications
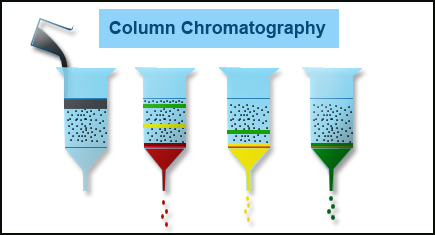
What is column chromatography?
Column chromatography is described as the useful technique in which the substances to be isolated are presented onto the highest point of a column loaded with an adsorbent (stationary phase), go through the column at various rates that rely upon the affinity of every substance for the adsorbent and the solvent or solvent mixture, and are typically gathered in solution as they pass from the column at various time.
The two most common examples of stationary phases for column chromatography are silica gel and alumina while organic solvents are regarded as the most common mobile phases.
Column Chromatography principle
The main principle involved in column chromatography is the adsorption of the solutes of the solution with the help of a stationary phase and afterward separates the mixture into independent components.
At the point when the mobile phase together with the mixture that requires to be isolated is brought in from the top of the column, the movement of the individual components of the mixture is at various rates.
The components with lower adsorption and affinity to the stationary phase head out quicker when contrasted with the greater adsorption and affinity with the stationary phase. The components that move rapidly are taken out first through the components that move slowly are eluted out last.
The adsorption of solute molecules to the column happens reversibly. The pace of the movement of the components is communicated as:
Rf = the distance traveled by solute/ the distance traveled by the solvent
Where Rf is called retardation factor
Column Chromatography Diagram (Image modified from https://bitesizebio.com/29947/basics-chromatography-column/)
Column Chromatography components
Components of a typical chromatographic system using a gas or liquid mobile phase include:
- Stationary phase – Generally it is a solid material having a good adsorption property and should be suitable for the analytes to be separate. It should not cause any hindrance in the flow of the mobile phase.
- Mobile phase and delivery system – This phase is made up of solvents that complement the stationary phase.
The mobile phase acts as a solvent, a developing agent (promotes separation of components in the sample to form bands), and an eluting agent (to remove the components from the column that are separated during the experiment).
- Column –
For liquid chromatography: 2-50cm long and 4mm internal diameter, fabricated with stainless steel
For gas chromatography: 1-3m long and 2-4mm internal diameter, fabricated either with glass or stainless steel
A column’s material and its dimension are very crucial to support the stationary phase and promote effective separations.
- Injector system – Responsible for delivering test samples to the column’s top in a reproducible pattern.
- Detector and Chart Recorder – This gives a continuous record of the presence of the analytes in the eluate as they come out from the column.
Detection relies on the measurement of a physical parameter (like visible or UV adsorption).
On the chart recorder, each separated analyte is represented by a peak.
A collector at the bottom is placed at the bottom end of the column set up to collect the separated analytes.
Column chromatography Procedure
The steps included in the column chromatography are:
- Preparation of the column
- Mostly the column is comprised of a glass tube with an appropriate stationary phase
- The bottom end of the column is packed with a glass wool/cotton wool or an asbestos pad after which the stationary phase is packed.
- After packing the column, a paper disc is placed on the top to avoid the disturbance of the stationary phase during the introduction of the sample or mobile phase.
The disturbance in the stationary phase (adsorbent layer) leads to the irregular bands of separation.
Two types of preparing the column, known as packing techniques namely:
- Dry packing technique – The amount of absorbent needed is added as a fine dry powder in the column and the solvent flows freely through the column until equilibrium is achieved.
- Wet packing technique – The slurry of adsorbent is prepared along with the mobile phase and is poured into the column.
It is regarded as the ideal technique for packaging.
The column should be properly washed and completely dried before in-use.
- Introduction of the sample
- The sample (a mixture of components) is dissolved in the minimum amount of the mobile phase.
- At one instant, the sample is introduced into the column and on the top portion of the column, it is absorbed.
- Through the elution process, the individual sample can be isolated from this zone.
- Elution technique
Through this technique, the individual components are separated completely from the column.
The process of elution can be carried out by employing two techniques:
- Isocratic elution technique – Throughout the procedure, a solvent of the same polarity or same solvent composition is utilized.
Example: Use of chloroform alone
- Gradient elution technique – Throughout the separation procedure, solvents of gradually increased polarity or increased elution strength are utilized.
Example: Benzene → Chloroform → Ethyl acetate → Chloroform
- Detection of Components
- In case the mixture separated in a column chromatography procedure are colored compounds, then monitoring the separation progress is simple.
- In case the compounds undergoing separation are colorless, then small fractions of the eluent are sequentially collected in tubes that are labeled. Thorugh TLC, the composition of each fraction is determined.
Types of Column chromatography
- Adsorption column chromatography – Technique of separation in which compounds to be separated (solute) is retained or adsorbed on the surface of the adsorbent (stationary phase).
- Partition column chromatography – It is based on the variance in partition coefficient of the individual components of the mixture, where the stationary phase and the mobile phase both are in the liquid state.
- Gel column chromatography – Here, the separation is carried out through a column packed with gel and possesses a porous stationary phase. It is also referred to as size exclusion chromatography
- Ion exchange column chromatography – The basis relies on the charge of the molecules. The separation is done when molecules get attracted to the oppositely charged stationary phase.
- Gas Chromatography (GC)
- High-Performance Liquid Chromatography (HPLC)
Column chromatography uses
Column chromatography is one of the versatile methods for purifying and separating both solids and liquids.
Major applications:
- To isolate active constituents
- To separate compound mixtures
- To remove impurities or carry purification process
- To isolate metabolites from biological fluids
- To estimate drugs in drug formulations or crude extracts
Sources:



Coloum Chromatography Topic Remember