How to select the optimum Laboratory Distillation Technique?

Distillation is a laboratory practice adopted to isolate a liquid component from others depending on differences in volatilities. Most of you would be familiar with simple or common distillation which is useful for isolation of a pure liquid from others when there is a significant difference between the volatilities. However, situations may arise when you have to adopt different distillation practices to effectively isolate the required component. The article reviews some of the commonly adopted distillation practices in routine laboratory separations.
Simple Distillation
The efficiency of separation is high when the boiling points of the liquids in the mixture are well separated (more than 25°C). It is a misconception that high purity of isolated component can be achieved in a simple distillation. In practice the distillate will still be a composite but with a higher proportion of the lower volatility component. For mixtures having vastly different boiling points (greater than or equal to 70°C) a single step distillation is generally sufficient.
The liquid mixture is heated to vaporize the lower boiling component and the vapour generated is condensed by passing through a water cooled condenser.
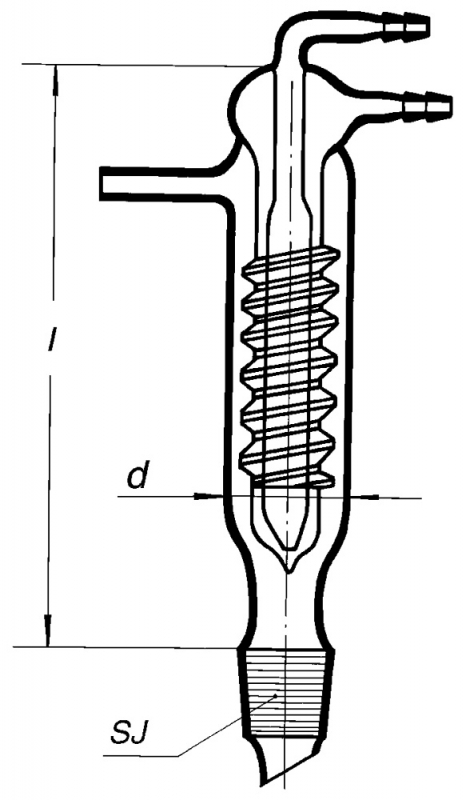
Fractional Distillation
Fractional distillation is resorted to when the liquids in the mixture have close boiling points. The vapour is passed through a fractionating column or condenser for repeated vaporisation – condensation cycles. The rising vapour condenses on the condenser walls and the surface of the packing material. The condensate gets reheated by the rising vapours and gets repeatedly vaporized.The cycle of condensation – vaporisation enriches the condensate with the lower volatility component and due to this process the distillation results in a higher purity of the separated component.
Steam Distillation
At times liquid mixtures can contain heat sensitive materials which can degrade on coming in contact with hot surfaces. In such cases steam distillation provides a better option. Steam is bubbled through the partially heated mixture. The resulting vapour is allowed to cool and condense yielding separate layers of water and oil such as in case of extracts of herbs and flowers and aromatic oils.
Vacuum Distillation
Some liquids require very high temperatures for boiling and distilling them at such temperatures could be impractical or even hazardous. The vapour pressure above the liquid surface gets reduced through application of vacuum resulting in preferential evaporation of low boiling components. The advantage is that distillation can be carried out at lower temperatures. The technique has almost replaced steam distillation in majority of applications. However, it is important to carefully examine the glassware for minor cracks as on application of vacuum dangerous implosions can occur. Fixing a tape on the glassware or use of wire mesh can prevent injuries due to flying glass pieces in the event of such accidents.

Rotary evaporation is an assembly that is practically useful for carrying out vacuum distillations. The round bottomed distillation flask rotates to spread the liquid on the walls of the flask to improve the evaporation rate and expedite the distillation.
Azeotropic Distillation
Conventional distillations cannot separate an azeotropic mixture whose solvent proportions are same as in vapour state In other words due to intermolecular attraction forces on boiling the components cannot be separated and their proportion remains unaltered.
A common example is a mixture containing 95.63% ethanol and 4.37% water. Ethanol boils at 78.4°C and water at 100°C under atmospheric pressure but the azeotropic mixture boils at 78.2°C which is lower than either of the two components. Azeotropic mixtures can be separated by resorting to azeotropic distillations.
In the above example the mixture can be shaken with calcium oxide which reacts instantaneously with water to form calcium hydroxide which is filtered out and the remaining filtrate is distilled to obtain pure ethanol.
Alternately a salt such as calcium acetate is dissolved in the liquid mixture to decrease the volatility of one component (water) and this helps break the azeotropic combination. Ethanol is unaffected and can be subsequently distilled over.
Extractive distillation can also be used where another solvent will form a separate layer and in which one of the components dissolves preferably and this helps distilling the other component. An example is adding water to 20% acetone and 80% chloroform azeotrope. Water will preferentially dissolve acetone and the distillate will be richer in chloroform.



Alaquainc corporations is specialized in the solvent distillation recovery method with the help of our world-class manufactured and designed solvent distillation equipment.