Which technique to use – GC or HPLC?


Chromatography has been used as a versatile technique in the laboratory for separation, identification and quantification of components in mixtures of organic compounds.
In the earlier stages Chromatography evolved from column chromatography and planar chromatographic separations based on paper chromatography and thin layer chromatography. Advances in instrumentation technology led to introduction of Gas Chromatography(GC) and High Performance Liquid Chromatography(HPLC). Today both GC and HPLC have gained a popular platform in modern analytical laboratories due to their versatility and scope of applications.
Both GC and HPLC are based on selective retention of sample components on the chromatographic column through specific interactions with the stationary phase followed by sequential elution and detection. The nature of elution varies greatly on chemical composition, solubility, molecular weight range and differences in polarity of the eluting compounds. You need to have clarity on the nature of components of the sample matrix, required levels of detection and also a clear understanding of the separation technique so that correct choice can be made. The focus of the present article is on differences between GC and HPLC techniques so as to help you decide the appropriate technique for the particular analysis in hand.
Mobile Phase
Mobile phase acts as a carrier of sample through the system. In HPLC the mobile phase is a liquid whereas in Gas chromatography it is a gas. Samples for both techniques should be liquids or solids which dissolve easily without leaving solid sediments (preferably mobile phase soluble in case of HPLC). Gaseous sample mixtures can be analyzed only by using GC.
Sample Stability
Thermal stability of a sample decides the choice of analysis technique. In GC analysis high temperatures in the range of 200°C to 400°C are commonly encountered at injection stage and in the separation column so the compounds present in the sample mixture should remain thermally stable at such temperatures. Thermally labile samples are analyzed using HPLC which is generally operated at room temperature.
Molecular Weight
Low molecular weight compounds are generally more volatile and are amenable to gas chromatographic detection. On the other hand high molecular weight compounds are less volatile and will not vaporize easily so such compounds are analyzed using HPLC technique.
Operating Pressure
Liquids encounter greater resistance to flow in columns in comparison to gases. It becomes necessary to use short and wide columns in HPLC. Gas chromatography in comparison uses much longer and narrow bore columns. Capillary columns have narrow id’s and length can be several meters. The operating pressure in GC is generally 150-200 psi whereas in analytical range HPLC separations it ranges from 2000 to 5000 psi which can be as high as 15,000 to 18,000 psi for Ultra High Pressure Chromatography systems
Nondestructive Analysis
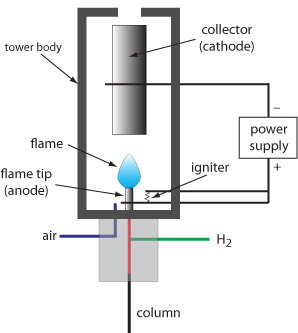
GC analysis generally uses destructive detection as in commonly used flame ionization detector. On the other hand in HPLC analysis the detection is based on nondestructive principles and if required the sample can be recovered. In the preparative mode the sample is isolated and recovered in measurable amounts depending on the scale of operation.

am sorry but am confused about application of HPLC and GC about the choise of which one of them
for GC must be highly volatile , thermal stable and low molecular weigh
is it true or what am ask u to do post about this please sir its confusing me
thanks sir
A post on this topic has already been published. Please see the link below. Hope it will clear some of your doubts.
https://lab-training.com/2017/04/27/technique-use-gc-hplc/
i think it is tru