Guidelines on Generation and Interpretation of Calibration Plots
Your choice of an analytical technique for a particular analysis would be based on its applicability and a conviction that the response will be linear to changes in concentration.
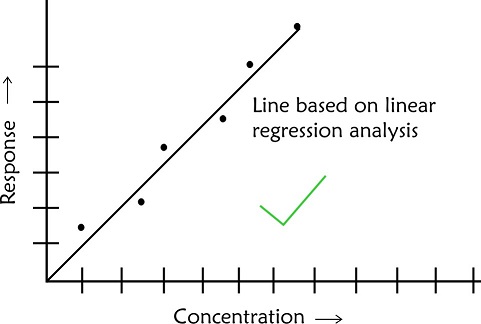
The graphical representation of concentration as x-axis and corresponding instrument response on y- axis is referred to as a calibration plot.
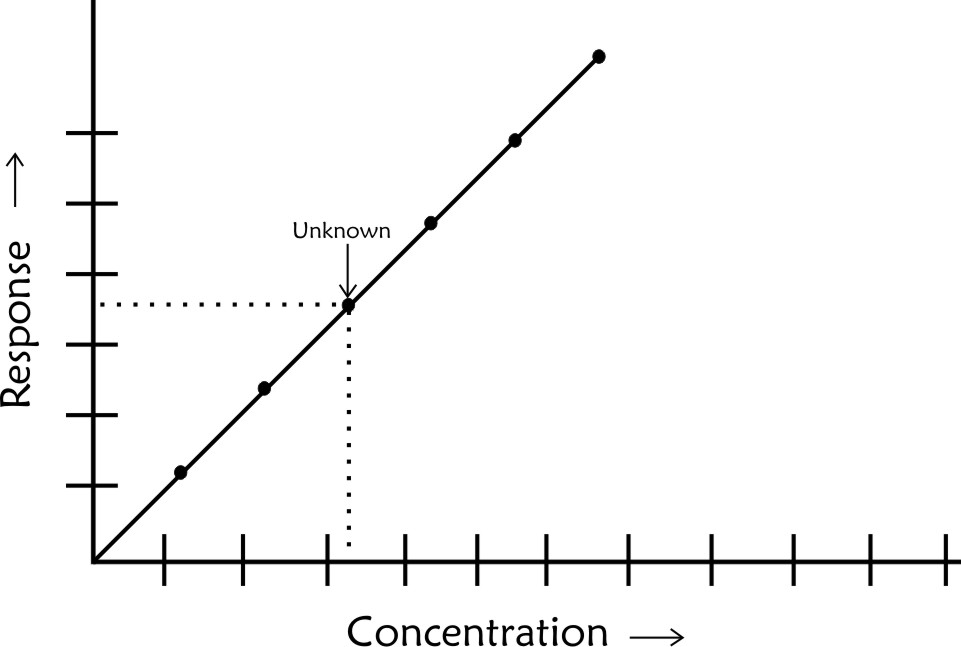
The concentration of unknown is determined by drawing a perpendicular to the concentration axis from the point of corresponding response. In addition to information on concentration of unknown a calibration plot provides additional information on
Linear Dynamic range which is the concentration range over which the technique gives a linear response to changes in concentration
Minimum detection limit (MDL) which is the minimum amount that can be detected but which cannot be quantified
Minimum detection quantity (MDQ) which is the minimum quantity that can be detected using the technique within the specified level of uncertainty. MDL is always lower than MDQ.
The objective of this article is to familiarize you with the calibration plot, information that you can gain from it and points to be kept in mind before making the calibration plot and its interpretation.
Essential considerations for establishing reliability of calibration plots :
- A calibration plot should be generated by using the traceable standard of the analyte whose concentration is to be determined.
- Maintain identical test conditions such as detector wavelength, sample matrix, chromatographic conditions, etc for all calibration point determinations
- Three points can establish a straight line but calibration plot should be made from at least 5 to 6 points which should include a blank determination.
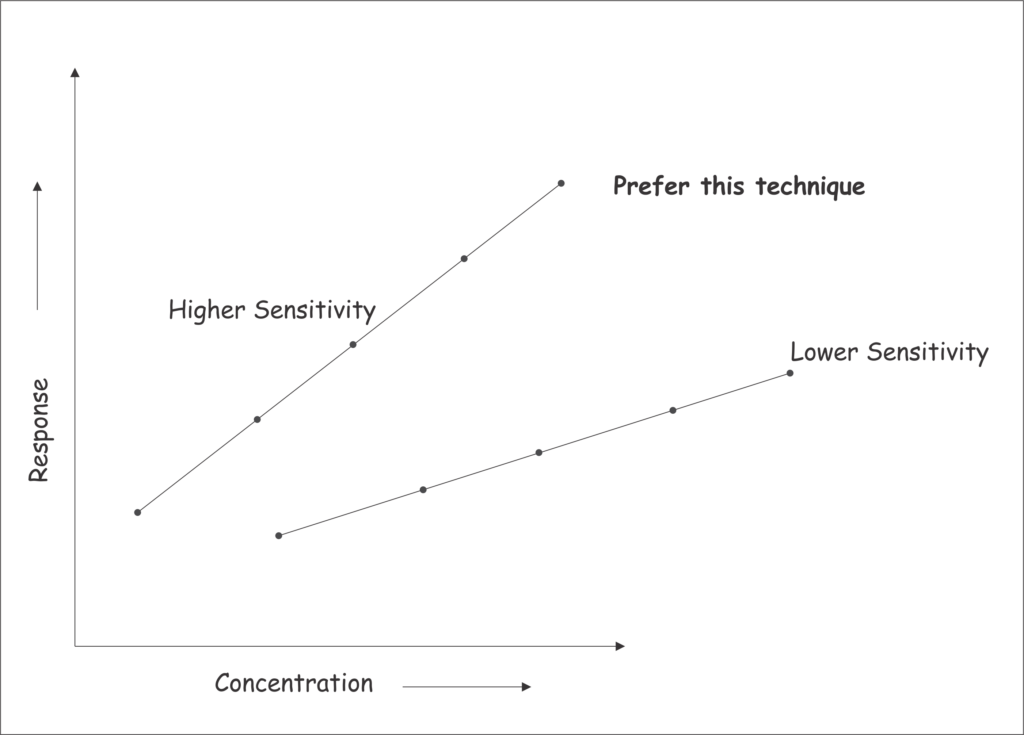
- Greater the slope of the calibration plot greater the sensitivity of the method as at lower concentrations the corresponding response signal will be higher than lower slope plots.
Mistakes to be avoided in generation of calibration plots
Some key guidelines are provided which you should verify before proceeding with interpretation and extrapolation of calibration plots
- A calibration plot should be generated from at least 5 to 6 points and should include a blank determination
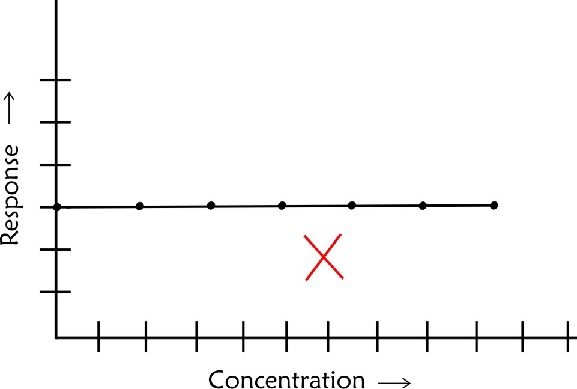
- If a calibration plot is a horizontal straight line it should be rejected straightaway as it would mean that the technique is not responding to changes in concentration of the sample
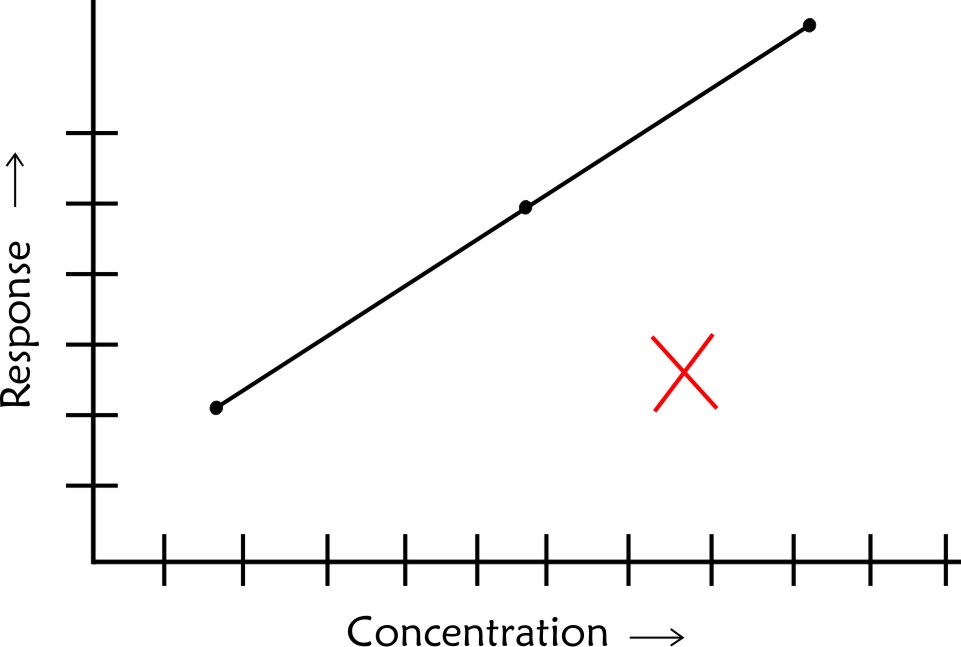
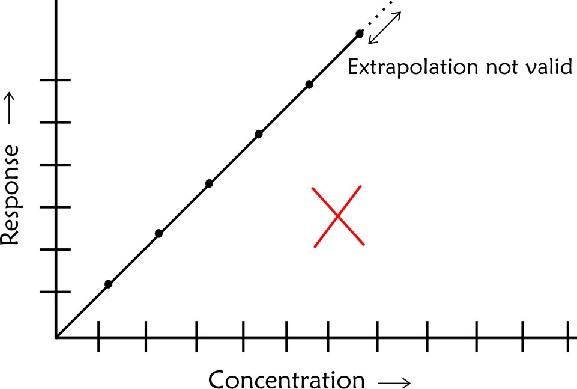
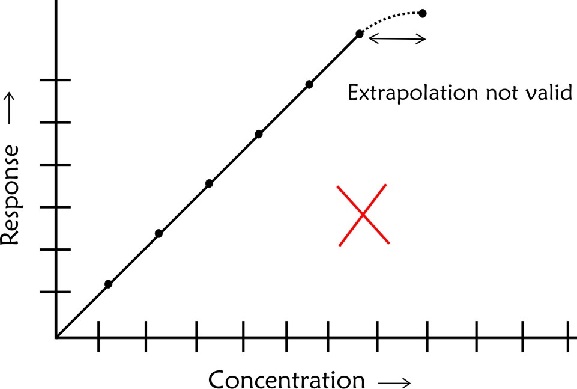
- The calibration line should not be extrapolated beyond the highest concentration recorded as you would not be sure about linearity beyond this point
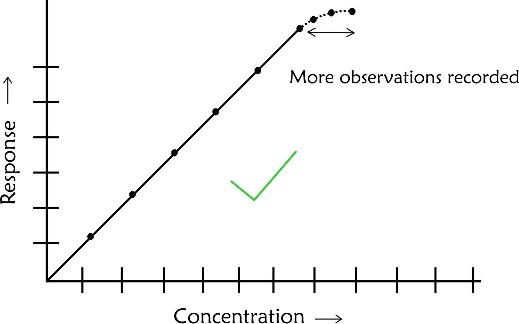
- If the highest point is not in a straight line with others do not attempt to draw a curve based on your judgement. In such case you need to have more points to establish the linear relationship beyond the last linear point
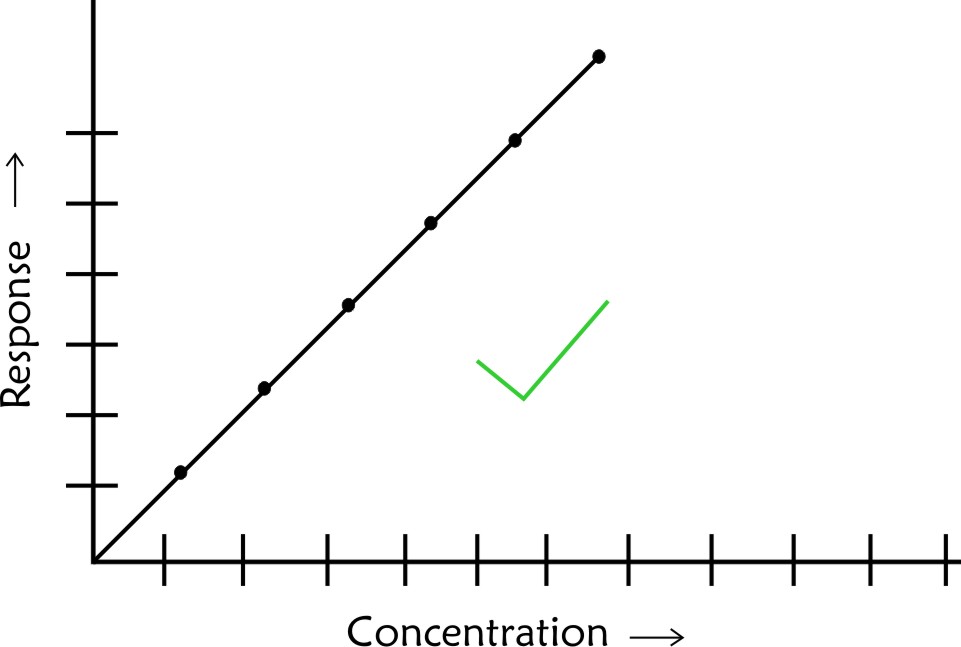
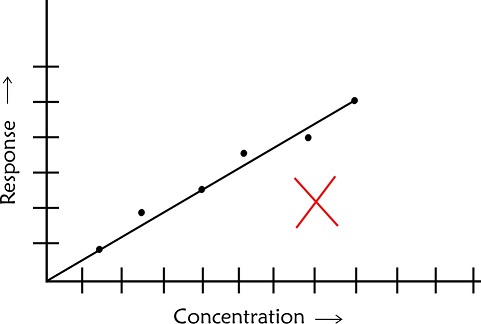
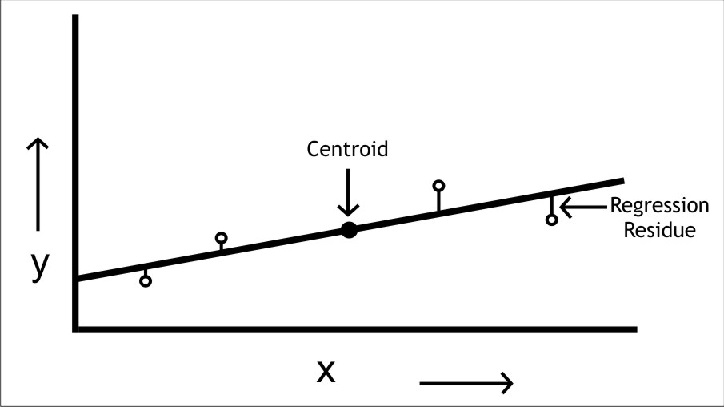
- In case of scatter of calibration points use linear regression analysis to establish a best fit line. Do not make personal judgement to decide on the best line which passes through the origin
In the present article linear plots have been covered but under real conditions deviations in linear behavior often take place above certain concentrations. The linearity is established on regression analysis and it is quantified using correlation coefficients. These aspects will be covered in a subsequent article.
Please let us know if you have liked the article and do leave your comments.



Please, always include me in your mailing list.
Thanks
Of course Please keep visiting the portal also for interesting articles and announcements
it was very helpful for me. thanks
[…] earlier article Guidelines on generation and interpretation of calibration plots dealt with considerations for establishing reliability of calibration. It also dealt with common […]
Good and very informative piece of article
Hi Kaushik,
Good to note that you found the article useful. We shall be open to your suggestions for improvements.
i relly learn from this link
I’m greatful about Dr Deepak’s post on the guid and step to ploting calibration graph. Unestly these steps he gave out have served as a guid for me on analytical chemistry which i am studying as a course currently in university, thank you so much Sir.
Thanks Kirian
Good analysis…
Thanks
Thanks, This information is very helpful.
very much informative with precise
The lecture is educative
Nice work!
but the addition of Beer Lambert’s law may be helpful to understand thoroughly
although there are certain limitations associated with Beer Lambert’s law, sometimes it may help to understand
i was very interesting on lab analytical experiment , please can you update me usually ?
Its quite comprehensive , especially for the beginners to understand concept linear plot and its contribution to quality of analyses. Thanks a lot Dr. Deepak, you are really giving a wonderful service to the analytical community.
Thank you doctor , it so intresting article