Advantages of direct solid sampling in Atomic Absorption Spectroscopy


Ever since its introduction as a versatile technique for trace metal analysis Atomic Absorption Spectroscopy has utilized liquid sample introduction as universally accepted mode of sample introduction. This mode supports analysis using both flame and graphite furnace atomization techniques.
Slurry sampling gained popularity with monitoring and control of manufacturing processes. However, it was accompanied with problems arising from clogging of nebulizers. Further the available time for vaporization in the flame is of the order of milliseconds which is insufficient for complete vaporization of larger size solid particles in slurries so graphite furnace atomization offered a viable option.
Introduction of solid samples direct into the Atomic Absorption Spectrometer became a reality towards the beginning of this century and gained acceptance with the availability of a commercial accessory. The automated accessory in conjunction with graphite furnace atomization holds promise over the conventional liquid sample introduction. The advantages offered are briefly covered in the article.
Time Saving
Samples received for analysis often comprise solids such as powders, soils, rocks, aggregates, inorganic salts, etc which after grinding for homogenization require chemical treatment such as solvent extraction and acid digestion which consume time. Introduction of solids directly can help reduce time taken by such processes. Automation affords precise sample introduction into the graphite furnace assembly which results in higher precision and accuracy of results.
Cost Saving
Bulk materials are often non-homogeneous so grinding to a fine powder introduces homogeneity. Afterwards sample pretreatment can require use of expensive solvents and chemicals to prepare the sample in liquid form for further analysis. Direct introduction of the powder can help save on the cost of such reagents and chemicals.
Freedom from harmful and toxic reagents
Use of toxic chemicals and corrosive acids accompanied with heating is not necessary if solid samples are directly introduced into the spectrometer in their original state. Further the risk of contamination of sample during processes such as digestion, weighing, serial dilutions, storage in volumetric glassware or vials prior to analysis is avoided. This eliminates contact or exposure to such harmful materials and protects the environment from discharge of such materials as laboratory wastes.
Freedom from contamination
As sample treatment is not necessary the sample does not get contaminated by impurities present in the laboratory reagents. The sample gets analyzed in its original state and the risk of contamination in sample preparation stages such as digestion, weighing, serial dilutions and during storage and handling is avoided.
Sample size
Availability of limited sample quantities can be a serious concern. In case of solids the problem can be easily overcome as analysis is possible for sample sizes as small as 100 μg to 10mg quantities.
Sensitivity improvements
Sample digestion results in dilution of the available sample. Losses can also result from storage of sample prior to analysis. The overall improvement of sensitivity can be around10 times compared with sample digestion or other sample pre-treatments.
The range of samples covers fine granules, fibers, creams and fine powders with precise sample introduction each time. However, complete homogeneity of sample needs to be ensured before introduction into the system.

 AAS, GC & HPLC Certificate Course Bundle
AAS, GC & HPLC Certificate Course Bundle  Certificate Course on AAS
Certificate Course on AAS  Certificate Course on GC
Certificate Course on GC  Certificate Course on High Performance Thin Layer Chromatography (HPTLC)
Certificate Course on High Performance Thin Layer Chromatography (HPTLC)  Certificate Course on ISO/IEC 17025:2017 - CPD Certified
Certificate Course on ISO/IEC 17025:2017 - CPD Certified  Certificate Course on Lab Safety
Certificate Course on Lab Safety  Certificate Course on Measurement Uncertainty in Chemical Testing
Certificate Course on Measurement Uncertainty in Chemical Testing  AAS, GC & HPLC Certificate Course Bundle
AAS, GC & HPLC Certificate Course Bundle  Certificate Course on AAS
Certificate Course on AAS  Certificate Course on GC
Certificate Course on GC  Certificate Course on High Performance Thin Layer Chromatography (HPTLC)
Certificate Course on High Performance Thin Layer Chromatography (HPTLC)  Certificate Course on ISO/IEC 17025:2017 - CPD Certified
Certificate Course on ISO/IEC 17025:2017 - CPD Certified  Certificate Course on Lab Safety
Certificate Course on Lab Safety  Certificate Course on Measurement Uncertainty in Chemical Testing
Certificate Course on Measurement Uncertainty in Chemical Testing 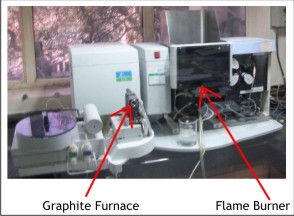


Responses