Prevention of Retention Time Drifts in HPLC Analysis
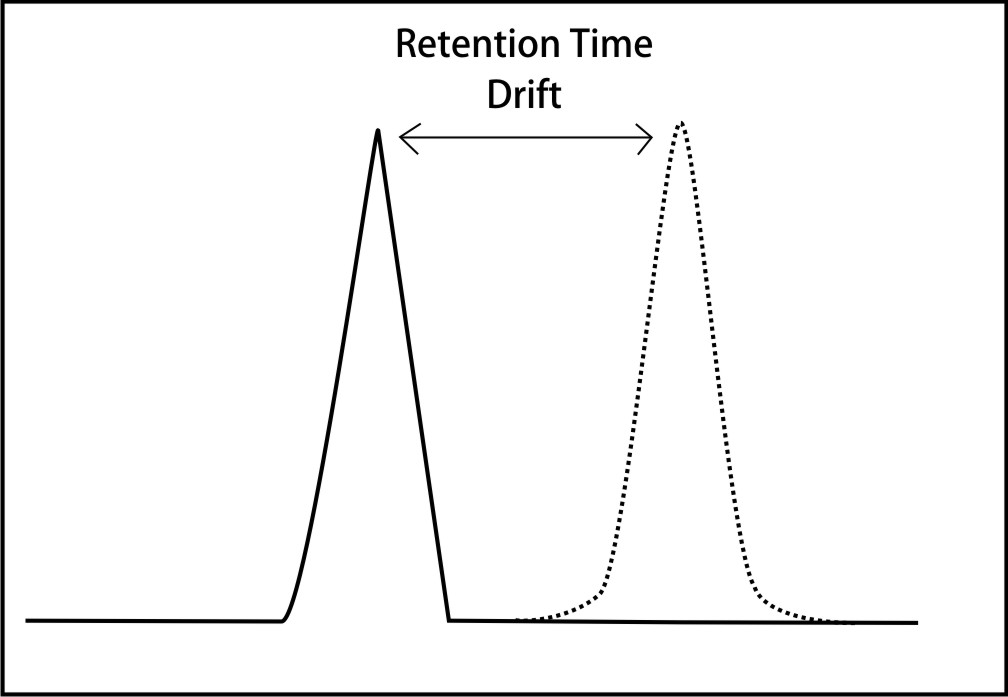
Retention time of a peak characterizes the identity of the eluting compound. Retention time has been explained in the earlier article How to read a chromatogram?. It is a vital analysis parameter and drifts resulting due to unintentional or uncontrolled changes in operational conditions can be annoying to the chromatographer. It is of utmost importance to maintain tight control over the operating conditions to prevent such drifts.

Retention time drifts are caused mainly by
- Changes in mobile phase composition
- Change in temperature of the column
- Change in surface groups of stationary phase
- Column to column variabilities
- Changes in mobile phase flow rate.
An understanding of reasons responsible for retention time drifts will help you appreciate the control measures.
Change in mobile phase composition
As the run using premixed mobile phase progresses some volatile organic components can evaporate thereby leading to variations in composition. Such losses can also take place if solvent bottles are not capped. Another reason for the losses is vacuum filtration of premixed solvents and degassing using ultrasonic baths which raise the temperature of mobile phase during sonication. Variations in concentration of premixed mobile phase can be avoided by using freshly prepared mobile phases, online degassing and keeping the bottles covered during use.
Changes in column temperature
Temperature changes affect both the mobile phase viscosity as well as retention mechanisms on stationary phase. Ionizable compounds tend to be affected more than non-ionizable compounds. Making use of column ovens which maintain the temperature of the column can reduce such drifts
Changes in surface groups of stationary phase
Surface groups tend to get affected by changes in buffer concentrations. Very low buffer concentrations are not able to maintain the required pH. Buffer concentrations above 0.1 M increase viscosity which can lead to back pressure problems. If lower than pH 2 media is used the stationary phase can get stripped of the organic functional groups.
Column to column variability
Column use history can result in measurable drifts in retention times. The retention times may not match with those on the column on which method was developed. Changes due to dwell volume of such columns can be offset by prolonging the equilibration of isocratic run before starting the main analysis.
Changes in flow rate
Changes in flow rate obviously result in drift in retention times. Lower rates lead to longer retention times for the same peaks. The flow rate consistency can be maintained by
- Maintaining column temperature by using column oven
- Checking for leaks around tubing connections, injection valves and regular inspection of damages to piston seals.
- Periodic inspection of check valves. Surface coatings formation on balls can lead to reduced flow. Clean or replace such balls.
- Back pressure fluctuations due to trapped air bubbles can be removed by system purging.
It can be concluded that retention time and drifts are caused by changes in mobile phase flow rate, mobile phase or stationary phase changes and column temperature. You are welcome to share your experiences and leave your valuable comments.



Sir, thank you for the article.
It was informative.
” If lower than pH 2 media is used the stationary phase can get stripped of the organic functional groups”-
this particular information was new for me.
Thanks Abhishek
Good to note that the article was useful.Special care should be taken in Reverse phase separations with regard to pH. It should be maintained between 2 and 8 to get long column life.
What is impact of diluent and insufficient column equilibration on retention time of analyte.
Hi Kishor, Both these factors can have varied effects on the retention time depending on the polarity and nature of solvents, column, mobile phase and analytes. For example THF as diluent can reduce RT for non polars and increase it for polars.
thanks sir for your article.
btw, can you teach me about value of drifting time. first the result is 4.3 and next analysis move to 4.4, i mean this moven is significant sir. do yo know rule to certain the peak is equal sir?
In reply to Kamella
See the response to Katrina provided by Dr Saurabh.Try to replicate your operating conditions for each run and this will surely narrow down the retention time difference.
Sir i am working on Xylitol production and i have tried HPLC for estimation with RID. When i inject standard in different concentrations peak shifted towards less retention time with lower concentration. What could be the base possible explanation for that?
Hi Vishal this might be because your sample diluent and mobile phase are different.