“Formal education will make you a living, self education will make you a fortune “
— Jim Rohn
In Module 1 an analogy was given comparing molecular mixture to a family passing by a candy store which is like the stationary phase. Children get retained because of their affinity for candy while the parents keep on moving like un- retained molecules leading to separation between them. Partitioning of sample molecules between a mobile phase and the stationary phase in the HPLC column is based on affinities which tend to hold back some molecules while allowing others to pass through freely.
The HPLC column stationary phase is where the separation occurs and is the most important part of the system. Different types of analysis are classified based on the type of stationary phase and mechanism behind the separation in the column.
The interactions are basically of three types:
- Polar Interactions
Differences in polarity between the sample components and the bonding entities on stationary phase result in preferential retention
- Ionic Interactions
Separation based on charge properties of sample molecules. Analyte ions have affinity for oppositely charged ionic centers on the stationary phase
- Molecular Size
Separation takes place due to entrapment of small molecules in the stationary phase pores. Large molecules pass through first followed by elution of smaller trapped molecules.
Now we shall briefly discuss the types of HPLC separations based on such interactions.
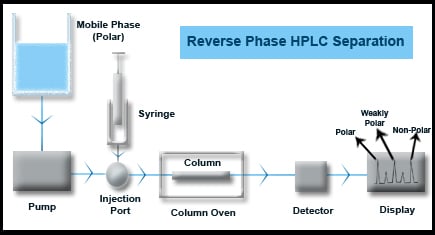
Reverse Phase HPLC Separations
Majority of HPLC applications are covered under reversed phase chromatography. Stationary phases mostly comprise of non polar alkyl hydrocarbons such as C-8 or C-18 chains bound to Silica or other inert supports. C18 columns actually can handle more than 60% of the applications in most HPLC labs. Mobile phase is polar and the elusion order is polar followed by less polar and weakly polar or non-polar compounds in the end.
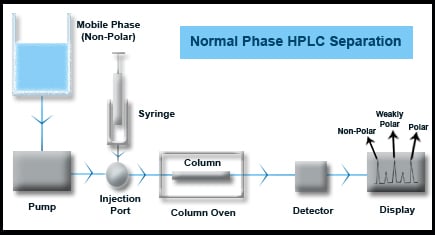
Normal Phase HPLC Separations
Normal Phase separations are the opposite of reverse phase separations. The stationary phases are polar having either plain silica or organic compounds such as amino, cyano, etc., groups bound to silica based supports. Mobile phases are non-polar such as hexane, heptane, etc. with small quantity of polar modifiers such as methanol, ethanol, isopropanol, etc. The elution order is non-polar molecules followed by weakly polar and polar molecules in the end.
Ion Exchange Chromatography
Synthetic organic resins are normally employed for separation or water soluble ionizable compounds. Anion exchangers have positive centres on surface and are used to separate compounds having sulfonate, phosphate or carboxylate groups. Cation exchangers have negative centers on the surface and are used to separate basic substances such as amines. Cross-linked styrene divinylbenzene is typical base material with charged groups linked to phenyl rings. Charges on packing material attract oppositely charged molecules from mobile phase and release them in inverse order of the attraction forces. Separation of components can be controlled by control of pH of mobile phase, temperature, ionic composition and addition of modifiers.
Size Exclusion Chromatography
Separation takes place on basis of molecular size of molecules. Small molecules get trapped in the stationary phase pores and exit after the large molecules. There are no chemical or ionic forces involved in the separation process. Such phases are available with silica or zirconium backbones with heavily cross-linked polymers and are used for separation of large molecules such as polysaccharides ,peptides, proteins and polymers.
Column length with the same stationary phase has significant effect on separation .Long and wide columns can take higher sample loads and provide higher resolution. On the other hand shorter columns reduce analysis time resulting in lower mobile phase consumption.
After an understanding of stationary phase it is necessary to understand mobile phases which primarily serve to carry the sample through the HPLC system.
Tip of the day
Never use a column as a stirrer and take care not to subject to mechanical shock as the packing can be irreversibly disturbed.
P.S
Did you get here from a link from a friend, or Twitter? This lesson is part 4 of 10 parts High Performance Liquid Chromatography Free e-course. To get more information about it and sign up Click here.