ICP-MS Interferences and their Removal
ICP-MS is a versatile and established technique for trace metal analysis for elements ranging from Li (Atomic mass 7) to Uranium (Atomic mass 250) in ppb, sub- ppb and even ppt levels. However, like any other trace metal analysis ICP-MS is also not free from interferences.
Types of Interferences
Isobaric Interferences
Isobaric interferences result due to presence of isotopes of same mass as the analyte element in the sample solution. Apart from elements in sample solution elements are also present as impurities in digestion acids or plasma gas and can contribute to isobaric interferences. The easiest approach is to make a correction by measuring the intensity of the interfering element from the intensity of the analyte isotope.
Polyatomic or Molecular interferences
Molecular interferences are due to a combination of sample or matrix ions with Ar and other matrix elements such as O, N, H,C,Cl,etc. Light elements such as Li, B or Be are not affected due to their small mass.
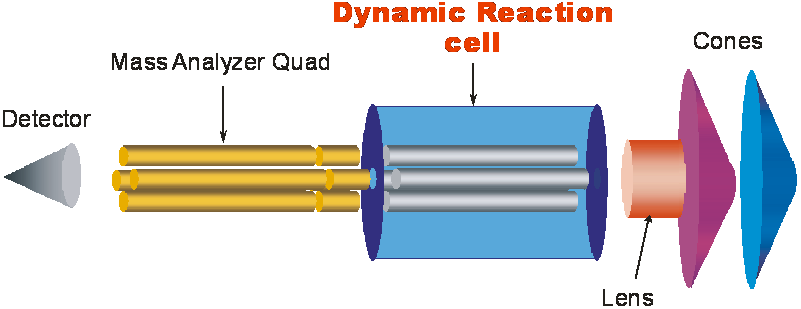
Starting with \(^3^9K\) this type of interference becomes significant for molecular species such as \(^3^9ArH\) and \(^2^3Na^1^6O\). The isotopes \(^5^6Fe, ^3^9K \) and \(^4^0Ca\) can all be interfered with Ar,O and N isotopes. For higher elements, lanthanides in particular, polyatomic interferences result from elements that are 16 atomic mass units lower due to metal-oxide formation. Polyatomic interferences generally cause problems in determination of first row of the periodic table due to large possibility of combination of Ar with matrix components. Collision cells offered by different manufacturers have reduced or even completely removed such interferences.A collision cell is located between ion optics and the mass analyzer. A quadrupole inside the reaction chamber is used to remove polyatomic interferences. Controlled reaction inside the collision cell such as ammonia converts the analyte of interest to another species resulting in removal of interference.
Doubly charged ion interference
Such interferences result from presence of doubly charged isotopes with twice the mass of analyte of interest. An example is interference from \(^2^0^6Pb^+^+\) upon \(^1^0^3Rh\) is likely at high Pb concentrations. Alkaline and rare earth elements form doubly charged ions to a greater extent than others. However, fortunately such interference is not common in Ar plasmas.
Space Charge Effects
A beam of positively charged ions is subject to mutual repulsion. Lighter elements with lower momentum are lost during lateral movement more than heavier elements. As a result the sensitivity of lower mass elements falls in presence of heavier mass elements. An example is loss of sensitivity of lithium in presence of high concentration of Uranium. On the other hand a high concentration of lithium will not depress Uranium concentration. Such space charge effects can be minimized by measuring the lighter element under reduced concentrations of heavier elements.
In general interferences in ICP-MS analysis can be overcome by :
- Sample purification for removal of species capable of producing polyatomic interferences.
- Use of blank correction for polyatomics produced by acid,water and plasma gases.
- Making use of collision cells with appropriate reaction gases
Hello!
Could you write a post on the most common interview questions in ICP-MS?
Thank you
Yes definitely but if you have any specific query please do communicate.